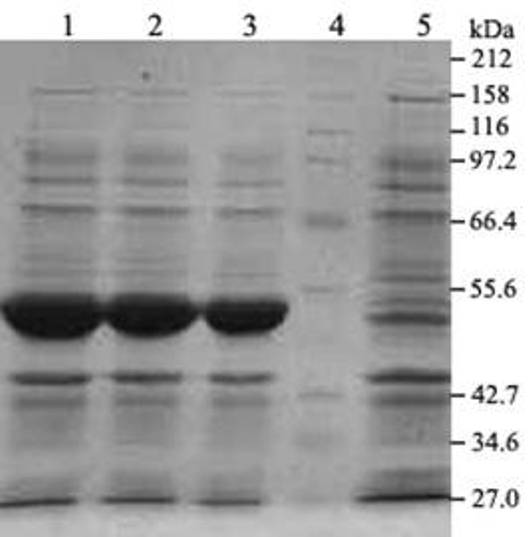
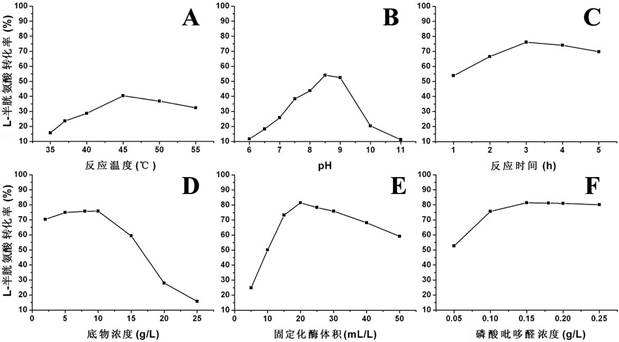
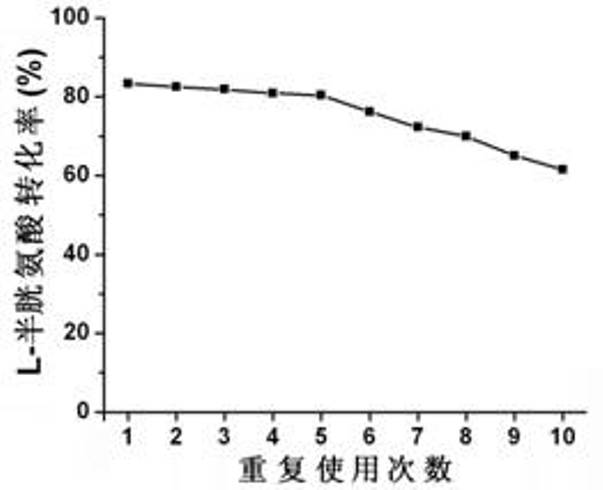
Method for synthesizing L-tryptophan by immobilized enzyme
A technology of immobilized enzyme and immobilized enzyme carrier, which is applied in the direction of fermentation, can solve the problems of difficult separation and high cost, and achieve the effects of high catalytic efficiency, easy separation and low fermentation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Construction of genetically engineered bacteria, induced expression of tryptophanase and preparation of crude enzyme solution
[0032] According to the tryptophanase gene sequence of Escherichia coli K12 strain, design upstream primer P1 of tryptophanase gene: 5'-CCG AAG CTT ATG GAA AAC TTT AAA CAT CTC C-3' and downstream primer P2: 5'-CCC GGA TCC TTA AAC TTC TTT CAG TTT TGC GG-3' Using the genomic DNA of Escherichia coli JM109 strain as a template, the tryptophanase gene was amplified by PCR, and the recombinant expression plasmid pET21a(+)-tnaA was constructed respectively.
[0033] The recombinant plasmid was transformed into Escherichia coli BL21(DE3), and the tryptophanase genetic engineering strain BL21(DE3)-pET21a(+)-tnaA was constructed.
[0034] Take a single colony of tryptophan engineered bacteria and inoculate it in LB medium, cultivate overnight at 37°C to obtain a saturated culture, inoculate the saturated culture at 1% in LB medium containing Amp (100 μ...
Embodiment 2
[0038] Determination of tryptophanase activity
[0039] In a 10 mL Erlenmeyer flask, add in order: 20 μL 0.2 mg / mL pyridoxal phosphate (PLP) solution, 10 μL 0.005 M reduced glutathione (GSH) solution, 270 μL obtained in Example 1 Tryptophan enzyme solution; 0.3 mL of the above solution was covered with 1 mL of toluene, incubated at 37 °C for 5 min, then added 100 μL of 5 mg / mL L-tryptophan solution, placed on a shaker at 37 °C for 10 min with gentle shaking , and then add 3 mL of indole chromogenic solution to stop the reaction, mix well, and measure the absorbance at 570 nm after standing for 30 min.
[0040] Definition of enzyme activity: under the above reaction conditions, the amount of enzyme required to catalyze the formation of 0.001 μmol indole at 37 °C for 10 minutes is an enzyme activity unit.
[0041] The expressed tryptophanase activity measured by the above method reached 3912.6 U / g, about 110 times of the tryptophanase activity of the original host bacteria itse...
Embodiment 3
[0043] Detection of L-Tryptophan in Reaction Solution by High Performance Liquid Chromatography
[0044] Immobilizing tryptophanase to obtain immobilized enzyme, using the immobilized enzyme as the enzyme source, using L-cysteine and indole as the substrate, at 35 to 55 °C, the pH value is 6 to 11, After enzymatic conversion reaction for 1 to 5 hours, L-tryptophan is produced. Remove the immobilized enzyme from the catalyzed reaction liquid, measure the concentration of tryptophan generated by high performance liquid chromatography in the filtered supernatant, compare it with the tryptophan standard curve, and calculate the L-cysteine in the substrate solution The molar conversion rate.
[0045] Chromatographic column: Nucleosil SA (250×4.6 mmol / L); injection volume: 20 mL.
[0046] Mobile phase: glacial acetic acid-triethylamine-water (0.1: 0.1: 99.8); flow rate: 1.0 mL / min; column temperature: 25 °C. UV detection wavelength: 280 nm.
[0047] ELSD detector parameter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com