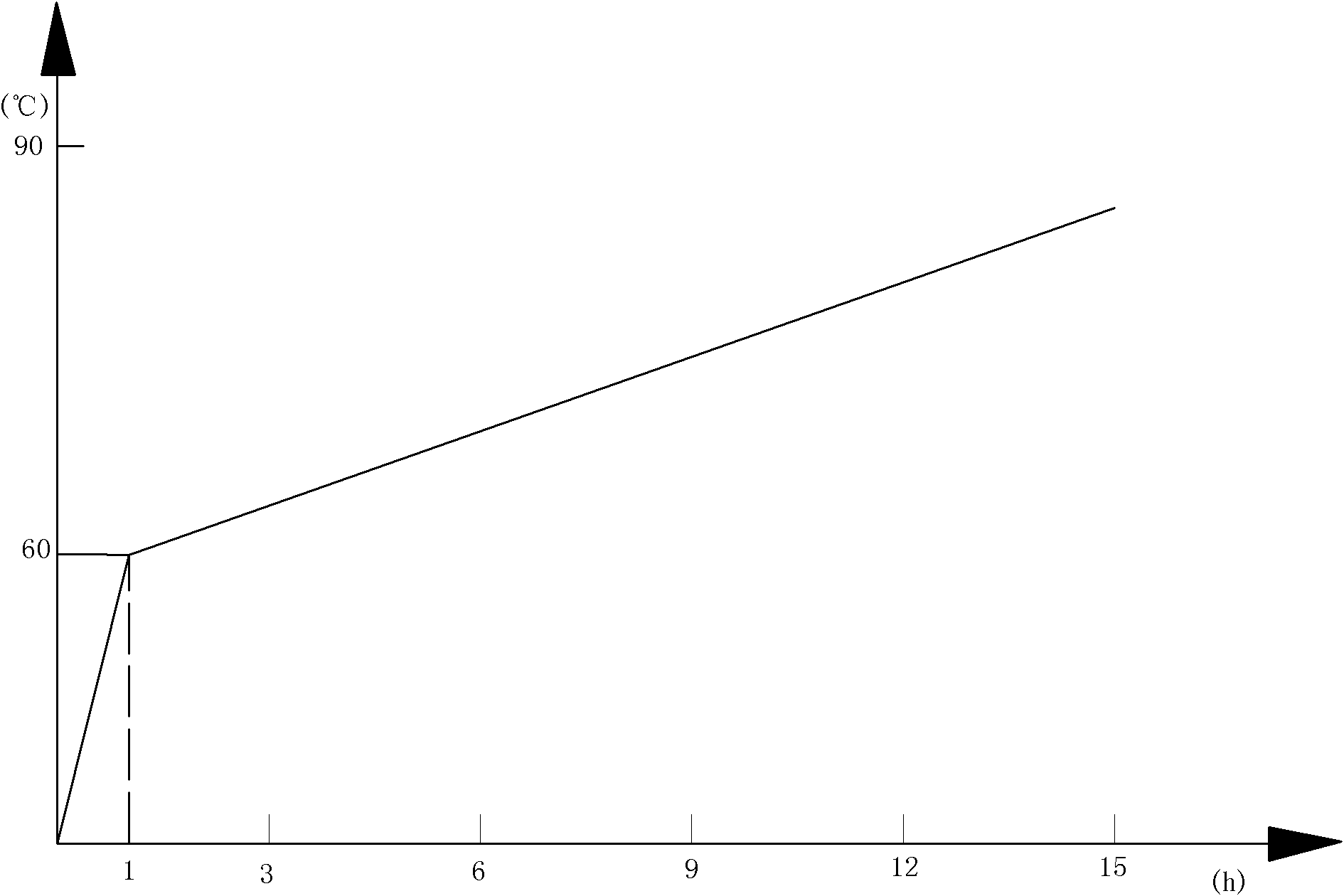Method for preparing p-fluoro anisole
A technology of p-fluoroanisole and fluoroanisole, which is applied in the field of preparation of high-purity p-fluoroanisole, can solve the problems that the reaction cannot be carried out, is not easy and economical, and cannot carry out condensation reaction, so as to ensure the production rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0028] The p-bromofluorobenzene in the raw materials used in this example was provided by Nanjing Fustar Chemical Co., Ltd., and the methanol solution of sodium methoxide was produced by Fengshui Social Welfare Chemical Factory, Zhangdian District, Zibo City, and its specification was 28wt%.
[0029] The preparation method of the p-fluoroanisole of the present embodiment may further comprise the steps:
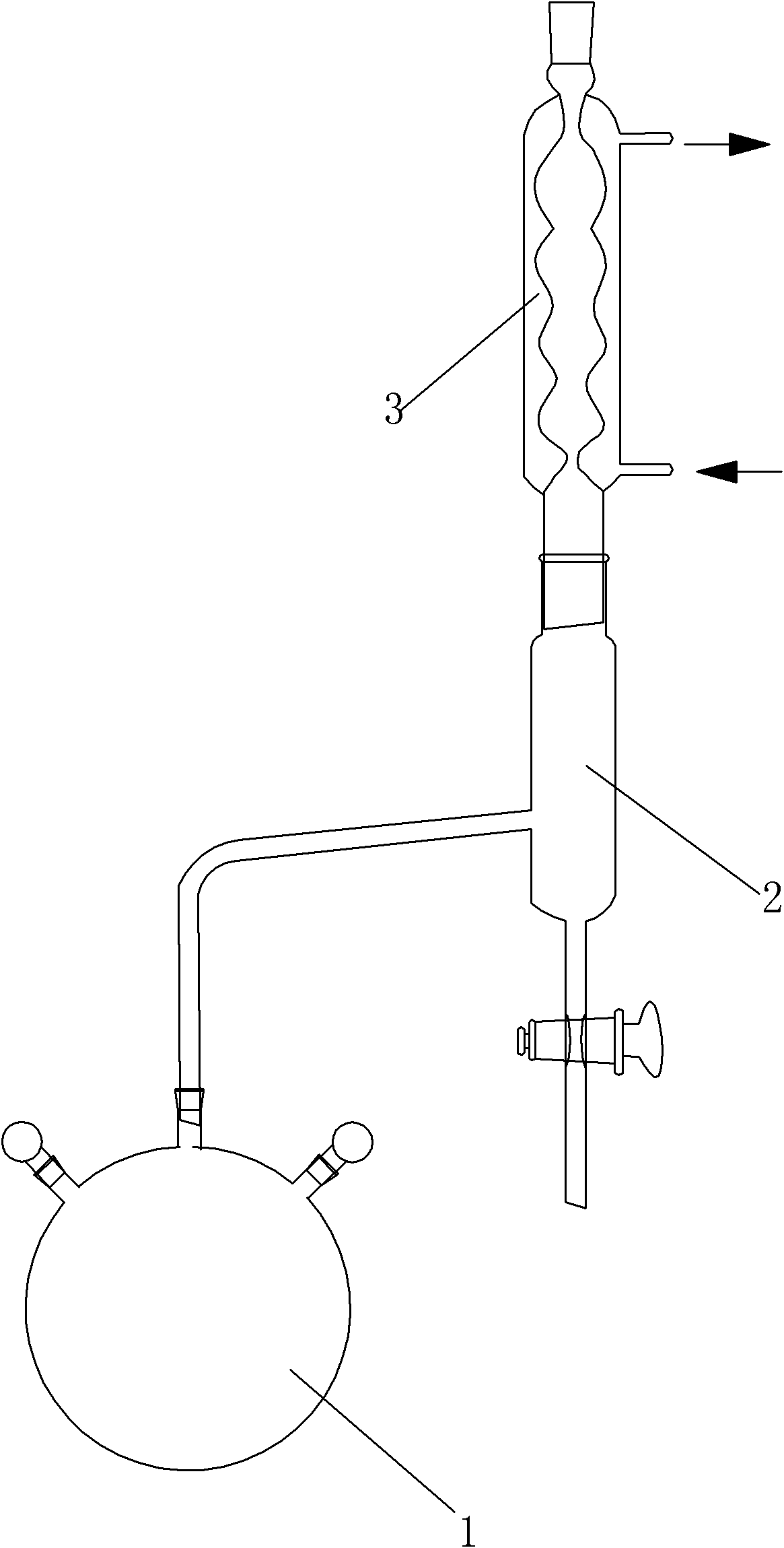
[0030] ①For the generation of p-fluoroanisole, add 500g (2.8mol) p-bromofluorobenzene and 260g solvent DMF ( The chemical name is dimethylformamide, which is a transparent liquid), 750g of 28wt% sodium methoxide in methanol (the amount of sodium methoxide is 3.92mol) and 8g of powdered catalyst cuprous chloride, on the head of the fractionation column A condenser is also installed, and cooling water is passed through the condenser;
[0031] Heat the above reaction system under stirring to make p-bromofluorobenzene and sodium methoxide react to generate p-fluoroanisole. During...
Embodiment 2)
[0071] All the other steps of the present embodiment are the same as in Example 1, except that in the step 1., when p-bromofluorobenzene reacts with liquid sodium methylate, the catalyst used is cuprous bromide, and the consumption of cuprous bromide is 10g.
Embodiment 3)
[0073] The rest of the steps of this embodiment are the same as in Example 1, except that in step 1., when the reaction system is heated under stirring to generate p-fluoroanisole, the reaction mixture is first brought from room temperature to 1. (20°C in this embodiment) slowly rise to 65°C, and then perform other operations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com