Method for synthesizing pyridinium hydroxy propyl sulfobetaine
A kind of hydroxypropanesulfonic acid pyridinium salt, the technology of synthesis method, applied in the field of synthesis of nickel plating brightener, can solve the problems such as difficult pH value and temperature control in the specified range, reaction conversion rate reduction, many by-products, etc., Achieve good catalytic reaction effect, reduce free radical polymerization, and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
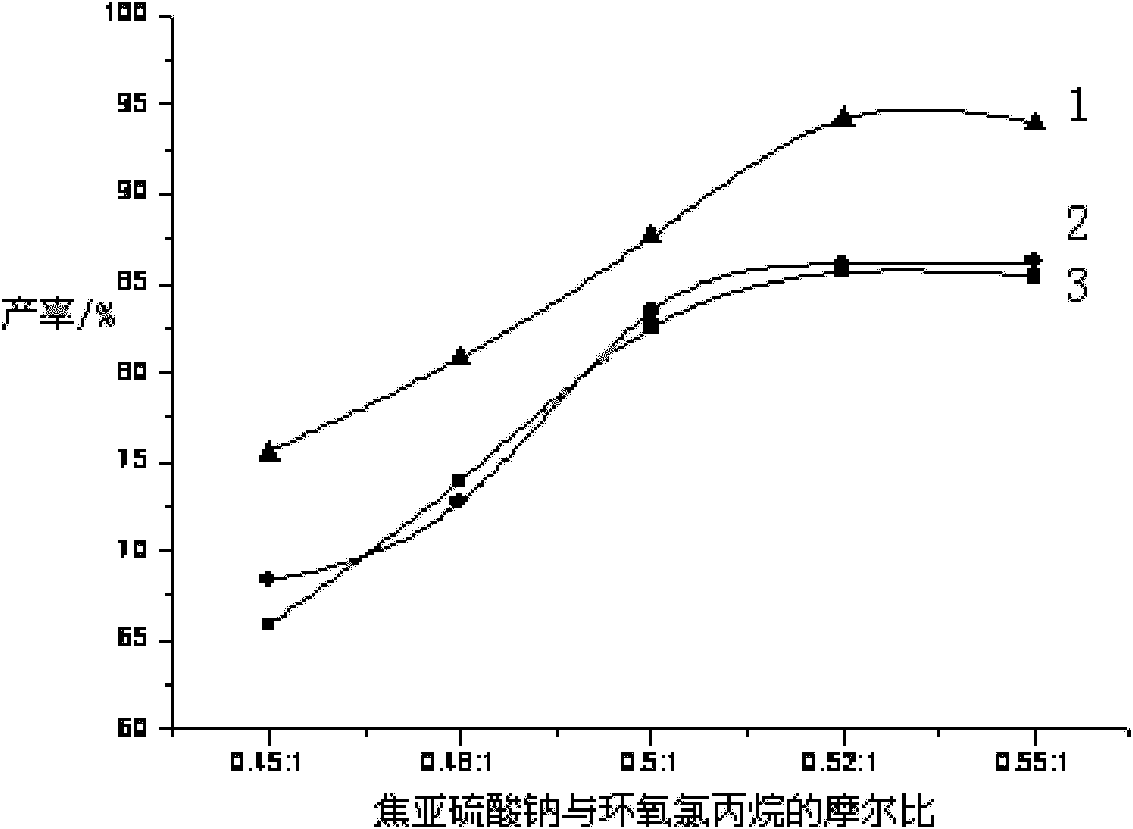
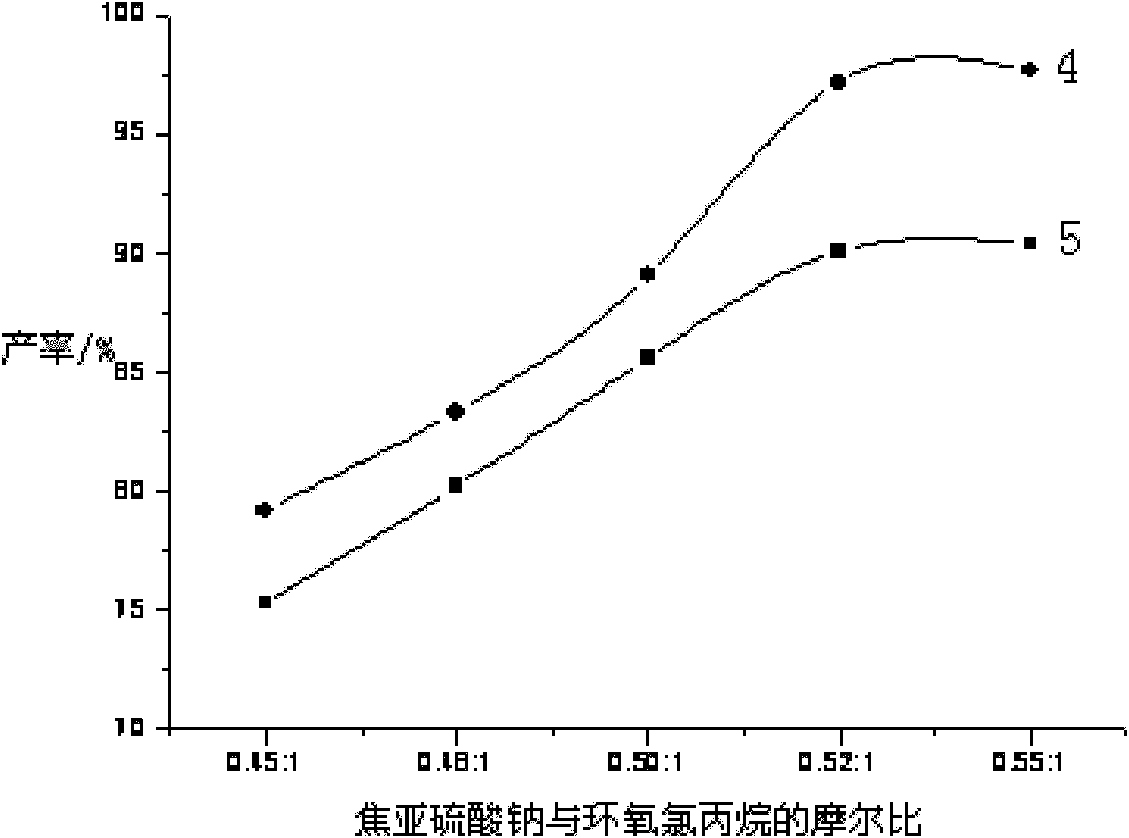
[0039] Add water (400g, 22.22mol), Na 2 S 2 o 5 (148g, 0.78mol), stirred to make it fully dissolved, then added 5.0gNa 2 SO 3. Pass in inert gas Ar, add 0.14g of phase transfer catalyst tetrabutylammonium bromide, raise the temperature to 40°C, slowly add epichlorohydrin (139g, 1.5mol) dropwise, and keep the reaction at 40°C for 2h after dropping, Then raise the temperature to 75° C. for 1 hour, and dehydrate under reduced pressure for 1 hour to obtain an aqueous solution of sodium 3-chloro-2-hydroxypropanesulfonate.
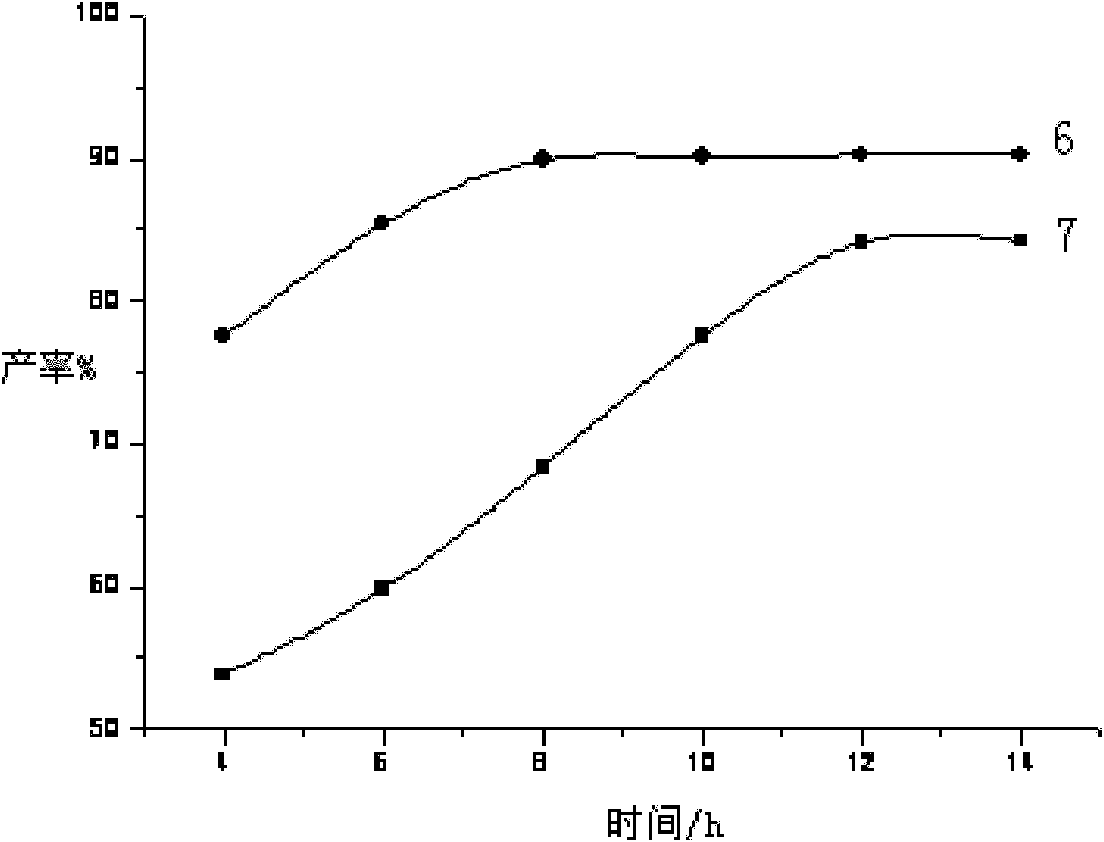
[0040] Pyridine (121g, 1.53mol) was directly added to the above solution, and 1.2g of high-efficiency catalyst 1,4,7,13-tetraoxa-10-aza-2,3-benzocyclopenta-2-ene was added, Raise the temperature to 30°C for 12 hours, dehydrate under reduced pressure for 3 hours, and decolorize to obtain PPS-OH liquid product 1 with a yield of 90.3%.
example 2
[0042] Add water (400g, 22.22mol), Na 2 S 2 o 5 (148g, 0.78mol), stirred to make it fully dissolved, then added 3gNa 2 SO 3 , add 0.14g of phase transfer catalyst triethylbenzyl ammonium chloride, heat up to 50°C, slowly add epichlorohydrin (139g, 1.5mol) dropwise, after dripping, keep warm at 50°C for 2h, then heat up to Insulate at 70°C for 1 h, dehydrate under reduced pressure for 1 h to obtain an aqueous solution of sodium 3-chloro-2-hydroxypropanesulfonate.
[0043] Pyridine (121g, 1.53mol) was directly added to the above solution, and then 1.2g of high-efficiency catalyst 1,4,7,13-tetraoxa-10-aza-2,3-benzocyclopenta-2-ene was added , heated to 40° C. for 11 h, dehydrated under reduced pressure for 3 h, and decolorized to obtain PPS-OH liquid product 2 with a yield of 91.6%.
example 3
[0045] Add water (400g, 22.22mol), Na 2 S 2 o 5 (148g, 0.78mol), stirred to make it fully dissolved, then added 5.0gNa 2 SO 3 . Pass inert gas N 2 , add phase transfer catalyst NP-21 (0.14g), raise the temperature to 55°C, slowly add epichlorohydrin (139g, 1.5mol) dropwise, keep the reaction at 55°C for 2h after dropping, then raise the temperature to 70°C and keep the temperature 1h, dehydration under reduced pressure for 1h to obtain 3-chloro-2-hydroxypropanesulfonate sodium aqueous solution.
[0046] Pyridine (121g, 1.53mol) was directly added to the above solution, and 1.2g of high-efficiency catalyst 1,4,7,13-tetraoxa-10-aza-2,3-benzocyclopenta-2-ene was added, Raise the temperature to 70° C. for 10 h, dehydrate under reduced pressure for 3 h, and decolorize to obtain PPS-OH liquid product 3 with a yield of 89.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com