Preparation method for agomelatine
A naphthyl and methoxyl technology, applied in the field of agomelatine preparation, can solve the problems of shortened production cycle, complicated reaction steps, harsh reaction conditions, etc., and achieve the effects of reducing reaction materials, shortening synthesis steps, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of reaction formula of preparing agomelatine described in the present invention is as follows:
[0027]
[0028] in:
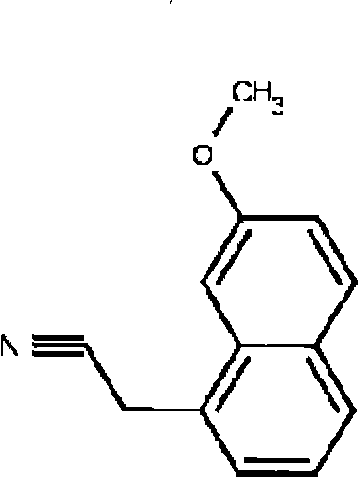
[0029] Formula 1 is (7-methoxy-1-naphthyl) acetonitrile
[0030] Formula 2 is 2-(7-methoxy-1-naphthyl) ethylamine
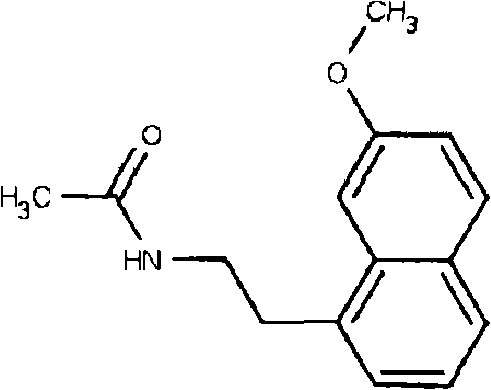
[0031] Formula 3 is N-[2-(7-methoxy-1-naphthyl) ethyl] acetamide
[0032] a is a reducing agent including: potassium borohydride, sodium borohydride, lithium aluminum tetrahydrogen, etc.;
[0033] b is nickel chloride hexahydrate; c is acetic anhydride
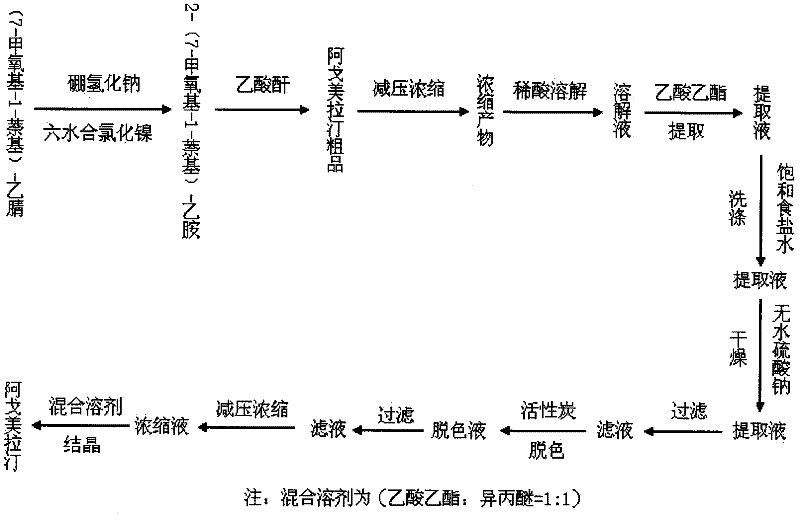
[0034] A flow chart of steps for preparing agomelatine according to the present invention is as follows figure 1 Shown (reducing agent is example with sodium borohydride).
[0035] In three 1L reactors, add 6.8g of (7-methoxy-1-naphthyl)acetonitrile, 5.1g of nickel chloride hexahydrate, 6.7g of acetic anhydride, and 150ml of methanol, and stir to dissolve at room temperature. Cool in an ice bath to -10°C, then add potassium borohydride, sodium borohydride, and lithium alumi...
Embodiment 2
[0037] In four 1L reactors, add (7-methoxy-1-naphthyl) acetonitrile 6.8g, nickel chloride hexahydrate 5.1g, acetic anhydride 6.7g, methanol 150ml, stir and dissolve at room temperature. Cool in an ice bath to -10°C, then add 2.6g, 3.9g, 5.2g, and 6.4g of sodium borohydride respectively, control the internal temperature of the reactor below 20°C, and stir at room temperature for 3 hours after the addition is complete. After completion of the reaction, concentrate the solvent to dryness under reduced pressure, add 100ml of ethyl acetate to dissolve the solid, then add 100ml of dilute hydrochloric acid with a concentration of 10%, extract the aqueous phase twice with 100ml of ethyl acetate after liquid separation, combine the organic phases, and wash with saturated salt Wash with water until nearly neutral, then add an appropriate amount of anhydrous sodium sulfate to dry; after drying, filter, add activated carbon to the filtrate and reflux for 30 minutes for decolorization. Act...
Embodiment 3
[0039]In three 1L reactors, add 6.8 g of (7-methoxy-1-naphthyl) acetonitrile, 5.1 g of nickel chloride hexahydrate, 6.7 g of acetic anhydride, and 150 ml of methanol, and stir to dissolve at room temperature. Cool in an ice bath to -10°C, add 5.2 g of sodium borohydride to the three reactors, strictly control the internal temperature of the reactors to not exceed 0°C, 10°C, and 20°C, respectively, and stir at room temperature for 3 hours after the addition is complete. After the reaction is complete, concentrate the solvent to dryness under reduced pressure, add 100ml of ethyl acetate to dissolve the solid, then add 100ml of dilute hydrochloric acid with a concentration of 10%, extract the aqueous phase twice with 100ml of ethyl acetate after liquid separation, combine the organic phases, and wash with saturated salt Wash with water until nearly neutral, then add an appropriate amount of anhydrous sodium sulfate to dry; after drying, filter, add activated carbon to the filtrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com