Method for extracting human fibrinogen from component I through column chromatography
A technology of human fibrinogen and column chromatography, which is applied to the preparation methods of fibrinogen and peptides, animal/human proteins, etc., can solve the problems of poor quality stability, low purity, and low yield, and achieve enhanced stability , to ensure stability and improve quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
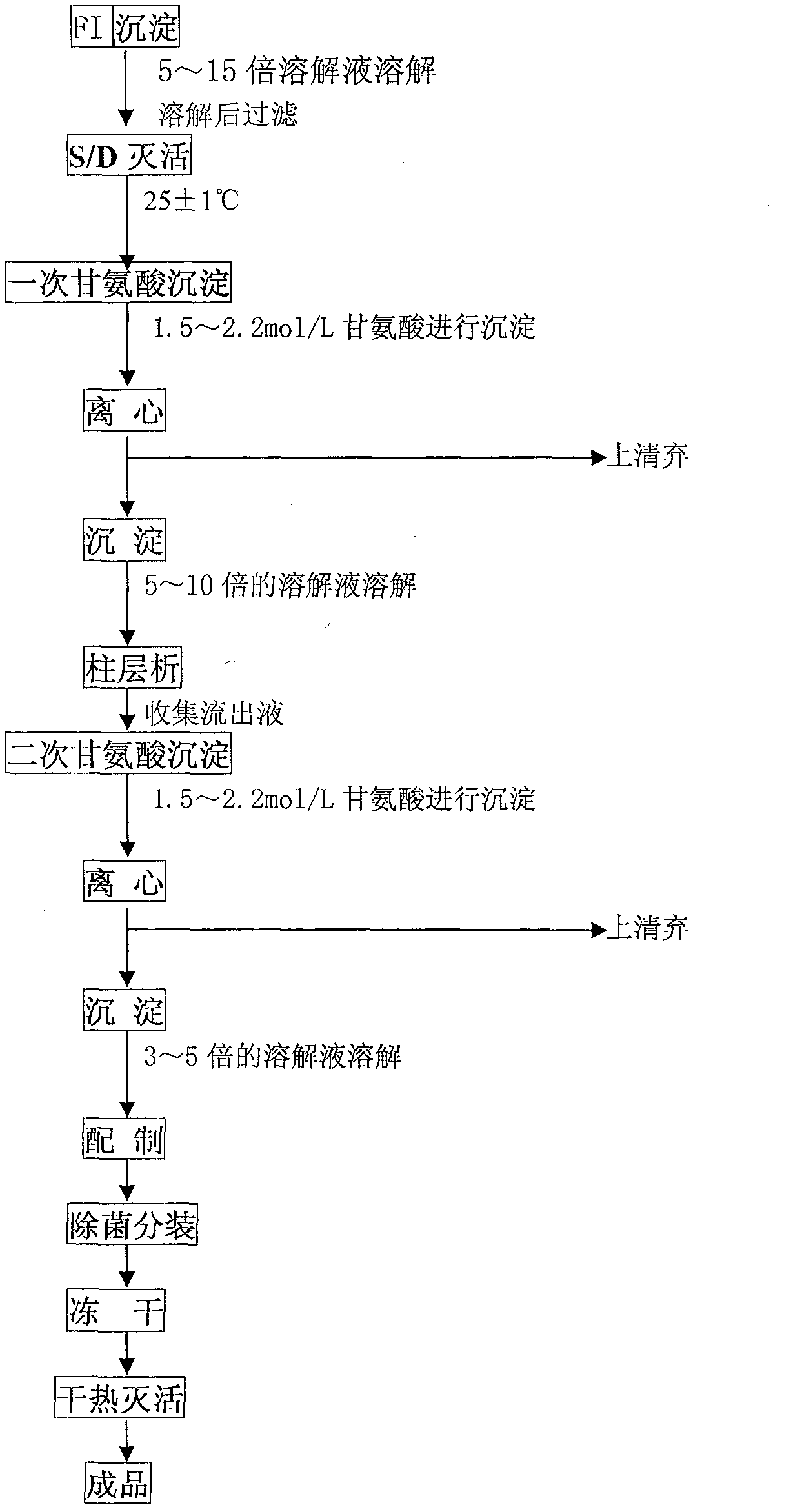
[0039] The present invention is realized by the following steps in concrete implementation:
[0040] (1) Dissolving and filtering of component I precipitation: use 5 times the volume of the first solution for the precipitation, stir and dissolve at 20°C for 1 hour, clarify and filter the dissolved product, and measure the volume of the product; the first solution is: Each 1000ml aqueous solution for injection contains: 10g trisodium citrate, 9g NaCl, 8g sucrose, 2g Tris, 3g lysine hydrochloride, adjust the pH value to 6.90 with HCl;
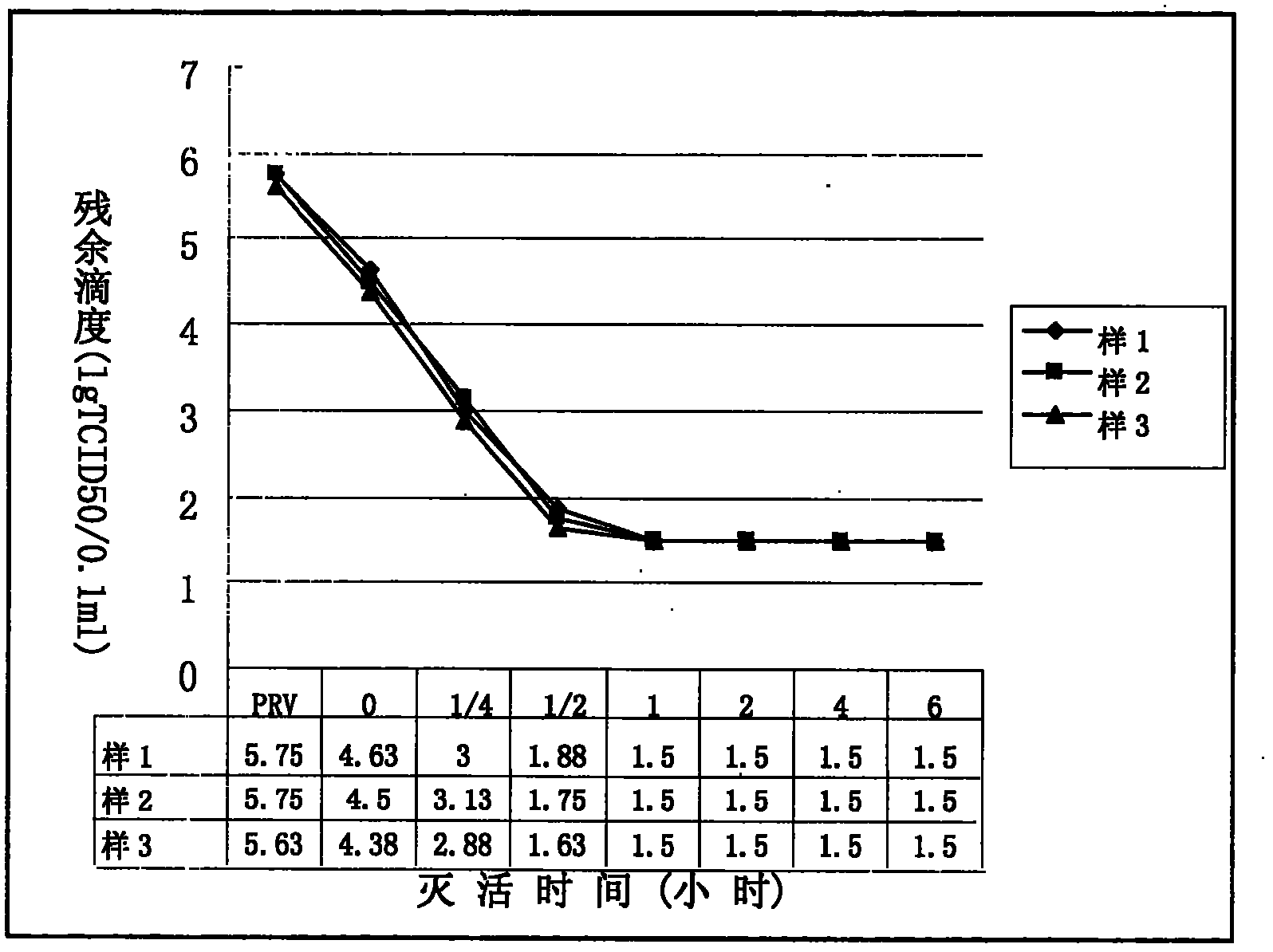
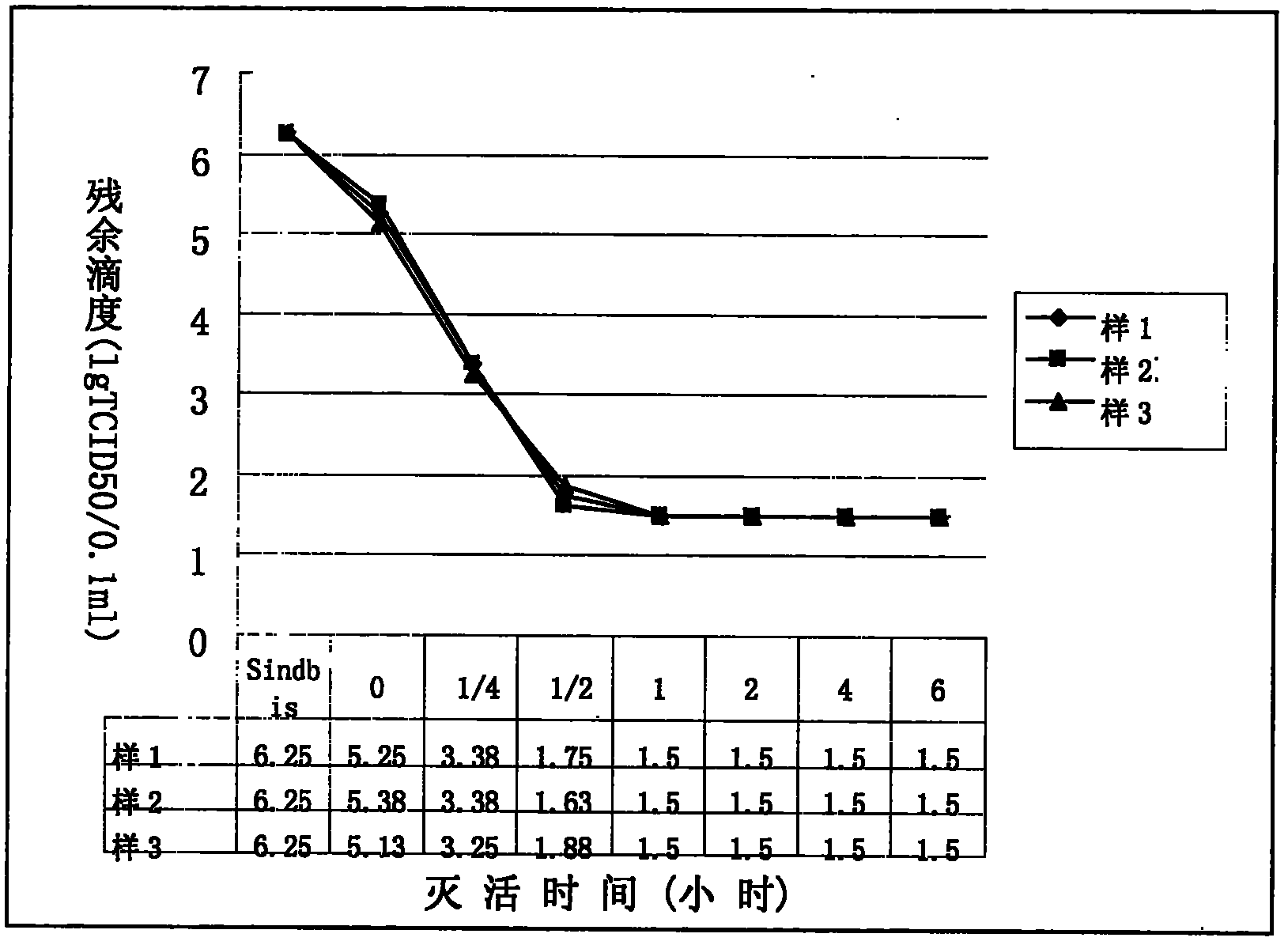
[0041](2) S / D inactivation: control the product temperature at 24°C, add 100ml per liter of filtrate, slowly add 11% S / D inactivator with a mass concentration under stirring, and keep the product temperature at 24°C6 Hour;
[0042] (3) The first glycine precipitation: measure the volume of the inactivation solution in step 2, add glycine powder at a concentration of 1.5mol / L, fully dissolve and cool to 2°C, perform continuous centrifugation at 4...
Embodiment 2
[0052] (1) Dissolving and filtering of component I precipitation: use 8 times the volume of the first solution for the precipitation, stir and dissolve at 23°C for 1 hour, clarify and filter the dissolved product, and measure the volume of the product; the first solution is: Each 1000ml aqueous solution for injection contains: 12g trisodium citrate, 9g NaCl, 10g sucrose, 3g Tris, 4g lysine hydrochloride, adjust the pH value to 7 with HCl;
[0053] (2) S / D inactivation: control the product temperature at 25°C, add 100ml per liter of filtrate, slowly add 11% S / D inactivator with a mass concentration under stirring, and keep the product temperature at 25°C6 Hour;
[0054] (3) The first glycine precipitation: measure the volume of inactivation solution in step 2, add glycine powder at a concentration of 1.8mol / L, fully dissolve and cool to 5°C, perform continuous centrifugation at 4200rpm for 40 minutes, and collect at 0°C precipitation;
[0055] (4) Dissolution and filtration o...
Embodiment 3
[0064] (1) Dissolving and filtering of component I precipitation: use 15 times the volume of the first solution for the precipitation, stir and dissolve at 26°C for 1 hour, clarify and filter the dissolved product, and measure the volume of the product; the first solution is: Each 1000ml aqueous solution for injection contains: 14g trisodium citrate, 9g NaCl, 12g sucrose, 4g Tris, 5g lysine hydrochloride, adjust the pH value to 7.10 with HCl;
[0065] (2) S / D inactivation: control the product temperature at 26°C, add 100ml per liter of filtrate, slowly add 11% S / D inactivator with a mass concentration under stirring, and keep the product temperature at 26°C6 Hour;
[0066] (3) The first glycine precipitation: measure the volume of inactivation solution in step 2, add glycine powder at a concentration of 2.2mol / L, fully dissolve and cool to 8°C, perform continuous centrifugation at 4200rpm for 40 minutes, and collect at 0°C precipitation;
[0067] (4) Dissolution and filtrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com