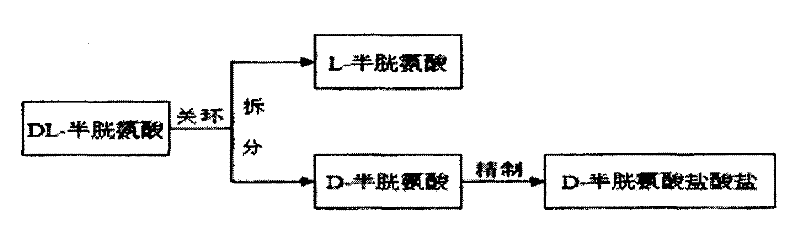
Method for preparing D-cysteine hydrochloride-hydrate
A technology of cysteine hydrochloride and cystine hydrochloride is applied in the field of preparation of D-cysteine hydrochloride monohydrate, which can solve the problem that the optical purity of the product cannot meet the requirements and the requirements of crystallization temperature Strict and difficult to select solvents, etc., to achieve the effects of easy control, less equipment investment, and easy production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
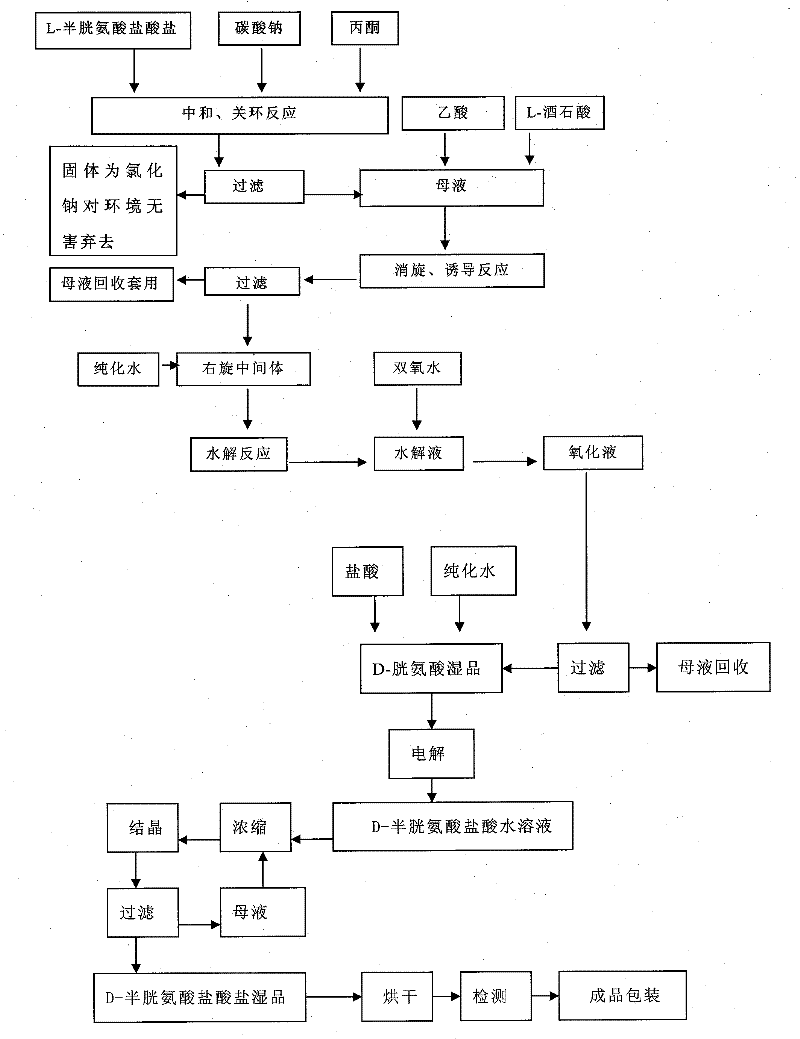
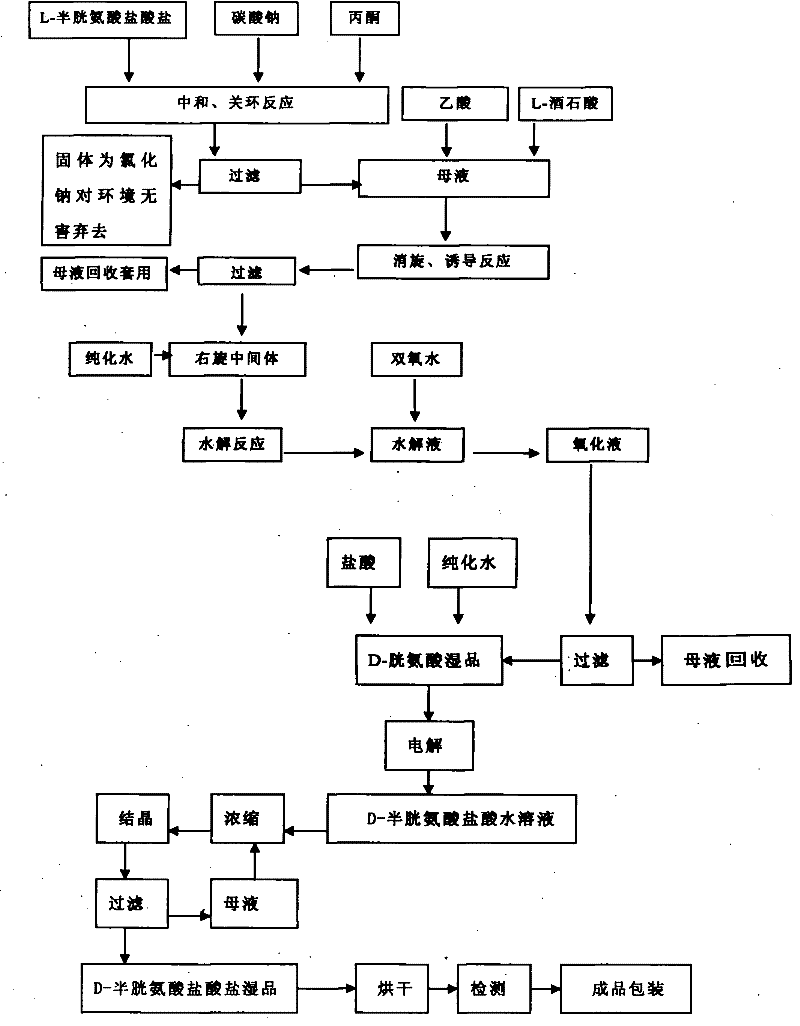
[0036] The preparation method of D-cysteine hydrochloride monohydrate adopts the following steps:
[0037] (1) 500 kilograms of acetone, 141 kilograms of L-cysteine hydrochloride and 47 kilograms of sodium carbonate were successively dropped into a reaction kettle equipped with a thermometer and an agitator, stirred and heated, reflux reaction at 60° C. for 5 hours, and cooled to 10 Filtrate at ℃, and reserve the mother liquor;
[0038] (2) Add 100 kg of anhydrous acetic acid and 120 kg of L-tartaric acid to the mother liquor in step (1), heat up at 50°C-55°C to induce racemization for 7 hours, cool down at 15°C-20°C and suction filter to obtain the dextrorotatory intermediate , filtrate recovery;
[0039] (3) Put 700 kg of purified water and 200 kg of dextrorotatory intermediate into the reaction kettle equipped with a thermometer and agitator successively, start stirring, heat up to 50°C, keep stirring for 4 hours, then cool down to 20°C-25°C, 50 kg of aqueous hydrogen...
Embodiment 2
[0042] The preparation method of the D-cysteine hydrochloride monohydrate adopts the following steps:
[0043] (1) Put 800 kilograms of acetone, 141 kilograms of L-cysteine hydrochloride and 55 kilograms of sodium carbonate into a reaction kettle equipped with a thermometer and an agitator successively, stir and heat up, reflux reaction at 55° C. for 4 hours, and cool down to 15 Filtrate at ℃, and reserve the mother liquor;
[0044] (2) Add 100 kg of anhydrous acetic acid and 120 kg of L-tartaric acid to the mother liquor in step (1), heat up at 50°C-55°C to induce racemization for 7 hours, cool down at 15°C-20°C and suction filter to obtain the dextrorotatory intermediate , filtrate recovery;
[0045] (3) Put 700 kg of purified water and 200 kg of dextrorotatory intermediate into the reaction kettle equipped with a thermometer and agitator successively, start stirring, heat up to 50°C, keep stirring for 4 hours, then cool down to 20°C-25°C, 50 kg of aqueous hydrogen per...
Embodiment 3
[0048] The preparation method of the D-cysteine hydrochloride monohydrate adopts the following steps:
[0049] (1) 500 kilograms of acetone, 141 kilograms of L-cysteine hydrochloride and 47 kilograms of sodium carbonate were successively put into a reaction kettle equipped with a thermometer and an agitator, stirred and heated, refluxed at 60°C for 5 hours, and cooled to - Filter at 5°C and reserve the mother liquor;
[0050] (2) Add 150 kg of anhydrous acetic acid and 120 kg of L-tartaric acid to the mother liquor of step (1), heat up at 50°C-55°C to induce racemization for 5 hours, cool down at 15°C-20°C and suction filter to obtain the dextrorotatory intermediate , filtrate recovery;
[0051] (3) Put 700 kg of purified water and 200 kg of dextrorotatory intermediate into the reaction kettle equipped with a thermometer and agitator successively, start stirring, heat up to 50°C, keep stirring for 4 hours, then cool down to 20°C-25°C, 50 kg of aqueous hydrogen peroxide s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com