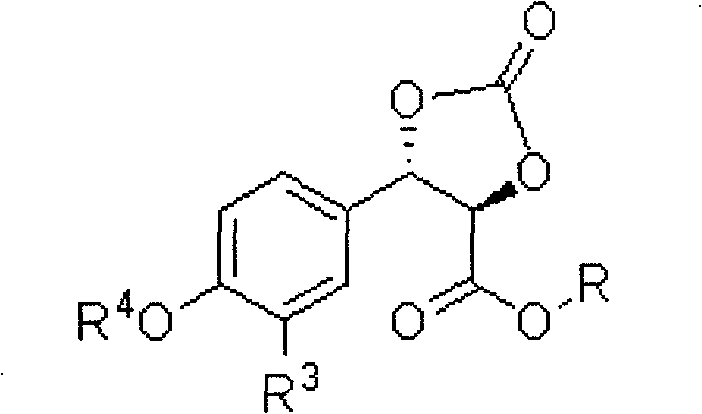
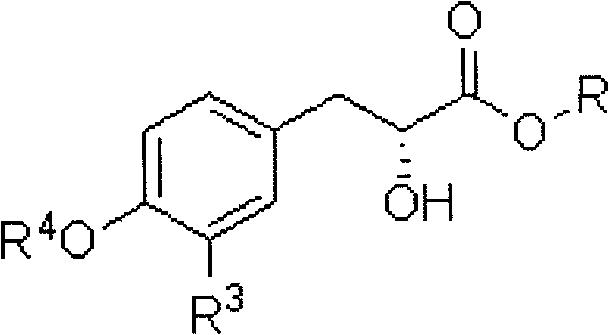
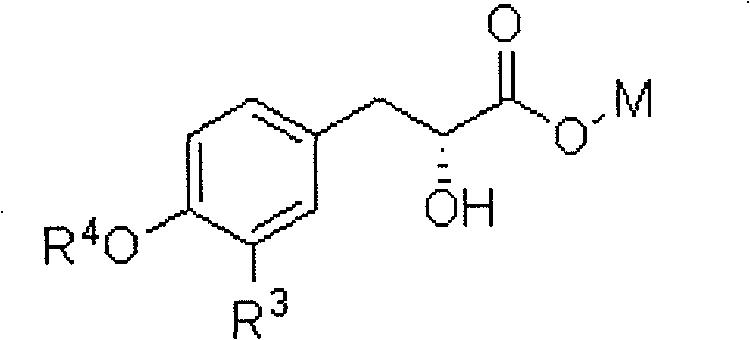
Preparation method of 3-aryl lactic acid derivatives
A technology of aryl lactic acid and aryl lactate, applied in the field of medicine, can solve the problems of narrow application range, complicated separation and purification, harsh conditions, etc., and achieve the effect of wide application range and high enantiopurity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Benzyl (2R,3S)-3-(3,4-dibenzyloxyphenyl)-3-hydroxylactate
[0038] 6.30 grams of 3-(3,4-dibenzyloxyphenyl) benzyl acrylate was dissolved in a mixed solvent of 34 milliliters of tert-butanol and 28 milliliters of dichloromethane, and 0.065 grams of (DHQ) were added successively at room temperature 2 -PHAL, 3.70 ml of 60% aqueous solution of N-methylmorpholine-N-oxide, and 0.010 g of K 2 OSo 2 (OH) 4 , stirred and reacted for 3 hours, TLC showed that the raw materials disappeared, added 2.50 g of sodium sulfite and 8 ml of water, stirred for 0.5 hours, filtered, washed the solid with dichloromethane, extracted the aqueous phase with ethyl acetate, combined the organic phases, washed with 1M hydrochloric acid , washed with brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated, and the residue was purified by flash column chromatography on silica gel, washed with ethyl acetate / petroleum ether=1 / 6 to 1 / 1, to obtain 5.00 g of product, 74% yi...
Embodiment 2
[0048] 1) Methyl (2R,3S)-3-(3,4-propylidenedioxyphenyl)-3-hydroxylactate
[0049] According to step 1 of Example 1, it was prepared from 3-(3,4-propylidenedioxyphenyl)-methyl acrylate. white solid.
[0050] 1 H NMR (CDCl 3 )6.84(d, 1H, J=1.8Hz), 6.78(dd, 1H, J=8.2, 1.5Hz), 6.64(d, 1H, J=7.9Hz), 4.90(m, 1H), 4.30(m, 1H), 3.80(s, 3H), 3.28(m, 1H), 2.89(m, 1H), 1.67(s, 3H), 1.66(s, 3H).
[0051] 2) Cyclocarbonate
[0052] According to step 2 of Example 1, it was prepared from (2R,3S)-3-(3,4-propylidenedioxyphenyl)-3-hydroxylactate methyl ester. Colorless oil.
[0053] 1 H NMR (CDCl 3 )6.70-6.50(m, 3H), 6.15(d, 1H, J=5.7Hz), 5.41(d, 1H,, J=5.6Hz), 3.70(s, 3H), 1.70(s, 3H), 1.68 (s, 3H).
[0054] 3) Methyl (R)-3-(3,4-propylidenedioxyphenyl) lactate
[0055] According to the hydrogenolysis reaction operation in Step 3 of Example 1, it was prepared from the cyclocarbonate obtained in the previous step, and purified by flash column chromatography on silica gel. Colorless o...
Embodiment 3
[0063] A kind of preparation method of 3-aryl lactic acid derivative, this method comprises the following steps:
[0064] (1) Cinnamate, N-methylmorpholine-N-oxide, 1,4-(dihydroquinineoxy)benzodiazine ((DHQ) 2 PHAL), potassium osmate by weight ratio is 400: 200: 6.5: 1 is placed in double hydroxylation reaction solvent, stirs 3 hours, through asymmetric double hydroxylation, prepares chiral diol, double hydroxylation reaction solvent is A mixture of tert-butanol and deionized water in a volume ratio of 1:1:1;
[0065] (2) Place the obtained chiral diol, acylating agent ditrichloromethyl ester, and organic base pyridine in the solvent tetrahydrofuran in a weight ratio of 10:4:3, and stir and react at room temperature for 40 minutes to prepare cyclocarbonic acid ester, the resulting cyclocarbonate structure is as follows:
[0066]
[0067] Wherein, R is C4 linear hydrocarbon group, R 4 for H, R 3 for H;
[0068] (3) cyclocarbonate, 10wt% palladium carbon is used as catal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com