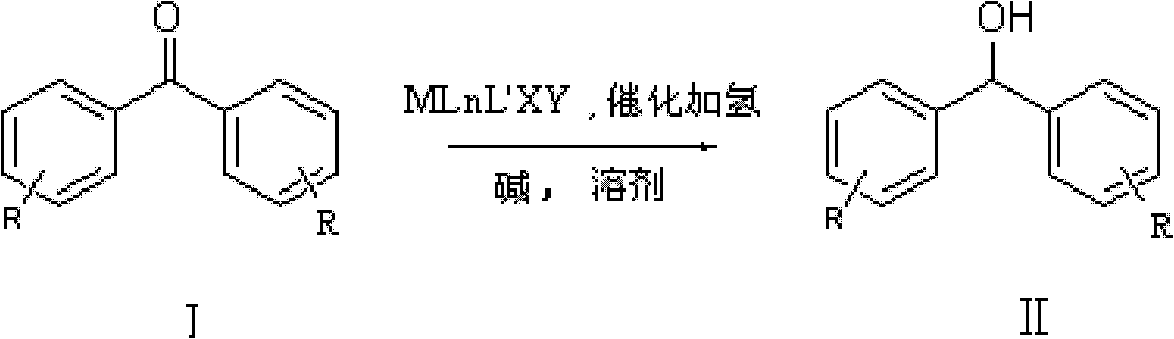Method for preparing diphenyl carbinol and derivatives thereof
A technology of diphenyl alcohol and its derivatives, which is applied in the field of preparation of diphenyl alcohol and its derivatives, can solve the problems of environmentally unfriendly metal waste liquid, cumbersome post-treatment, low yield, etc. The effect of short cycle and stable reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
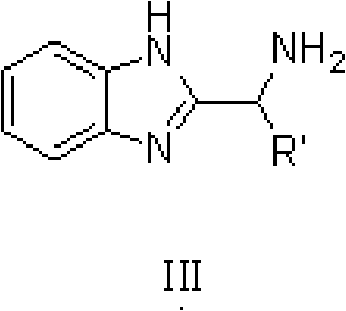
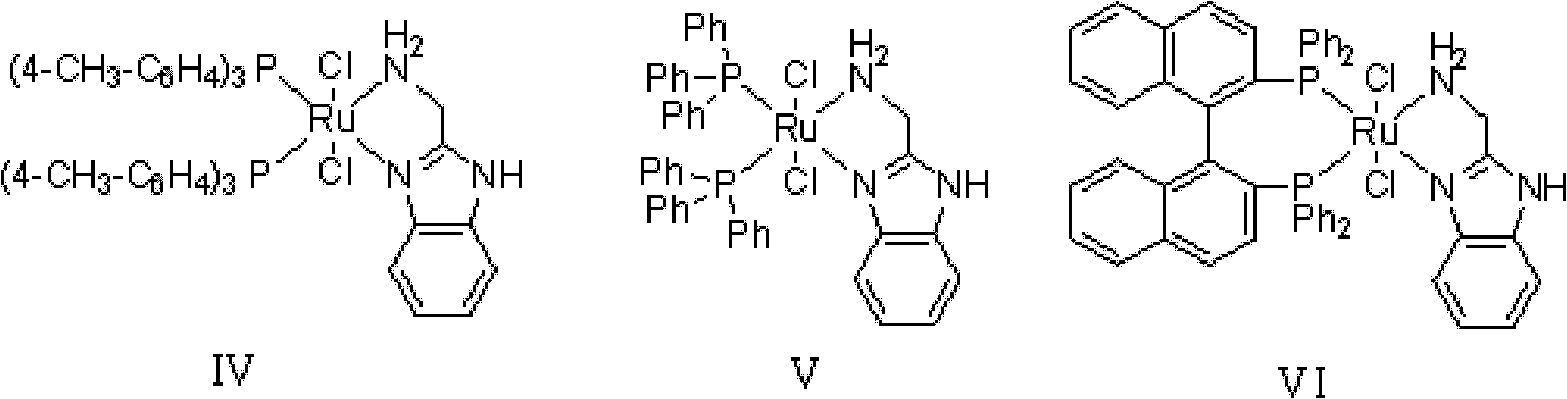
[0035] In 100L stainless steel reaction kettle, add benzophenone, toluene, in N 2 Atmosphere, add catalyst trans-[RuCl 2 (PPh 3 ) 2 {(1H-benzo[alpha]imidazol-2-yl)methanamine}] and potassium tert-butoxide; filled with H 2 To 5 atm, 100 ℃ of stirring reaction, when the hydrogen pressure is constant (about 4 hours), stop stirring, the H in the reactor 2 Empty, sample the reaction solution, carry out conventional post-processing (filtration, centrifugation, filtration, extraction, concentration, drying, etc.) to obtain a white or off-white solid product, which is detected by gas chromatography. The conversion rate of the reaction is 99.2%.
Embodiment 2
[0037]
[0038]
[0039] In 100L stainless steel reaction kettle, add benzophenone, tetrahydrofuran, in N 2 Atmosphere, add catalyst trans-[RuCl 2 (PPh 3 ) 2 {(1H-benzo[alpha]imidazol-2-yl)methanamine}] and triethylamine; filled with H 2 To 10atm, 30 ℃ of stirring reaction, when the hydrogen pressure is constant (about 8 hours), stop stirring, the H in the reactor 2 Vent, sample the reaction solution, and perform conventional post-treatment to obtain a white or off-white solid product, which is detected by gas chromatography, and the conversion rate of the reaction is 99%.
Embodiment 3
[0041]
[0042] In the 100L stainless steel reaction kettle, add benzophenone, dichloromethane, in N 2 Atmosphere, add catalyst trans-[RuCl 2 {P(C 6 h 4 -4-CH 3 ) 3} 2 {(1H-benzo[alpha]imidazol-2-yl)methanamine}] and sodium tert-butoxide; filled with H 2 To 60atm, 25 ℃ stirring reaction, when the hydrogen pressure is constant (about 18 hours), stop stirring, the H in the reactor 2 Vent, sample the reaction solution, and perform conventional post-treatment to obtain a white or off-white solid product, which is detected by gas chromatography, and the conversion rate of the reaction is 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com