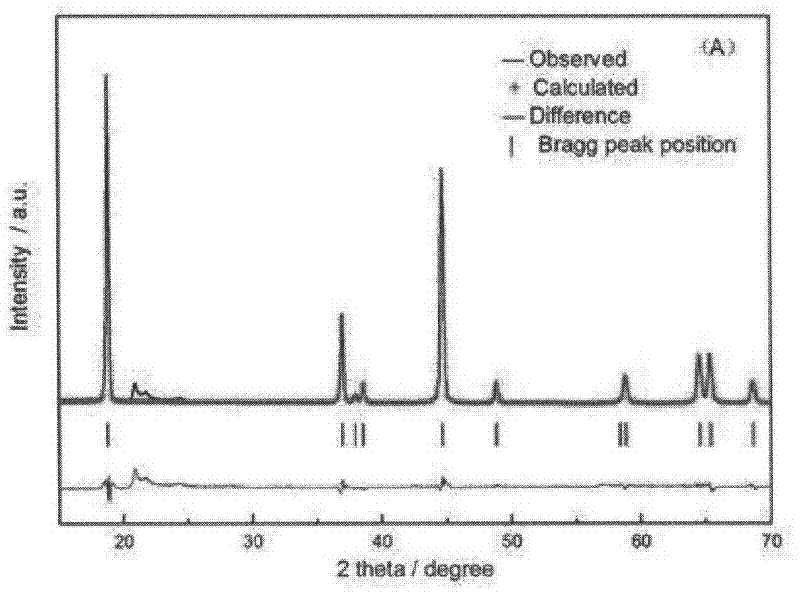
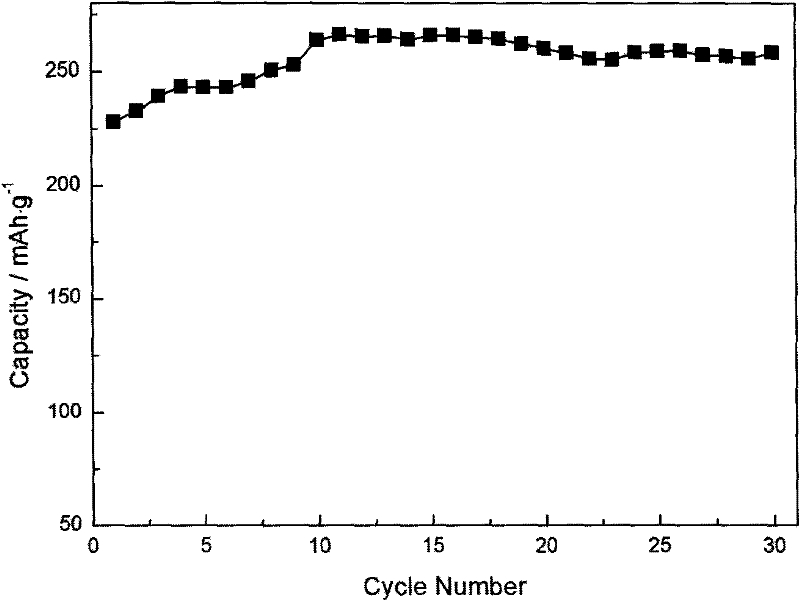
Method for preparing lithium-enriched cathodic material of lithium ion battery
A lithium-rich positive electrode material, lithium-ion battery technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of poor electronic conductivity and ion conductivity, low initial charge and discharge efficiency of materials, and poor high-current discharge performance, etc. problems, to achieve the effect of improving the rate and cycle stability, small fluctuations in physical and chemical properties, and uniform coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Implementation Example 1: Preparation of lithium-rich cathode material Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 , namely Li[Li x mn y m (1-x-y) ]O 2 (x=0.2, y=0.2, M=Mn 0.5 Ni 0.5 ).
[0025] Dissolve a certain amount of nickel nitrate, a mixture of manganese nitrate (the ratio of nickel ions to manganese ions is 1:1) and ammonium oxalate in water respectively at room temperature. Evenly drop the ammonium oxalate solution and the mixture solution into the reactor, mix well, react, then seal the reactor, place it in an oven at 180°C, take it out after 12 hours, and cool it naturally to form a co-precipitation of oxalate with uniform distribution of nickel and manganese . The oxalate precipitate was washed several times with deionized water, filtered, and dried. Grind the dried precipitated powder with a certain amount of lithium carbonate evenly, pre-fire at a constant temperature of 450°C for 6 hours, press it into a sheet after cooling, and calcinate at 850°C for 12 hou...
Embodiment 2
[0029] Implementation Example 2: Preparation of lithium-rich cathode material Li[Li 0.2 mn 0.54 co 0.13 Ni 0.13 ]O 2 , namely Li[Li x mn y m (1-x-y) ]O 2 (x=0.2, y=0.4, M=Mn 0.5 Ni 0.5 co 0.5 ).
[0030] Dissolve a certain amount of a mixture of nickel sulfate, cobalt sulfate and manganese sulfate (wherein the ratio of nickel ion, cobalt ion and manganese ion is 1:1:1) and sodium oxalate in water at room temperature. Drop the sodium oxalate solution and the mixture solution evenly into the reactor, mix well, react, then seal the reactor, put it in an oven at 200°C, take it out after 12 hours, and cool it naturally to form a cobalt oxalate with uniform distribution of nickel, cobalt and manganese. precipitation. The oxalate precipitate was washed several times with deionized water, filtered, and dried. Grind the dried precipitated powder with a certain amount of lithium carbonate evenly, pre-fire it at a constant temperature of 500°C for 5 hours, press it into a sh...
Embodiment 3
[0032] Implementation Example 3: Preparation of lithium-rich cathode material Li[Li 0.2 mn 0.4 co 0.2 Ni 0.2 ]O 2 , namely Li[Li x mn y m (1-x-y) ]O 2 (x=0.2, y=0.2, M=Mn 0.5 Ni 0.5 co 0.5 ).
[0033]Dissolve a certain amount of a mixture of nickel sulfate, cobalt sulfate and manganese sulfate (wherein the ratio of nickel ion, cobalt ion and manganese ion is 1:1:1) and sodium oxalate in water at room temperature. Evenly drop the sodium oxalate solution and the mixture solution into the reactor, mix well, react, then seal the reactor, place it in an oven at 150°C, take it out after 18 hours, and cool it naturally to form a cobalt oxalate with uniform distribution of nickel, cobalt and manganese. precipitation. The oxalate precipitate was washed several times with deionized water, filtered, and dried. Grind the dried precipitated powder with a certain amount of lithium carbonate evenly, pre-fire it at a constant temperature of 550°C for 4.5 hours, press it into a sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com