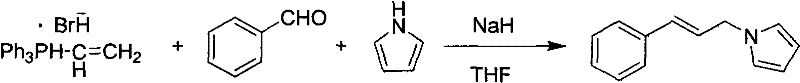
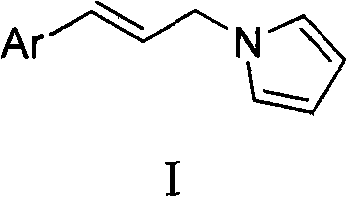
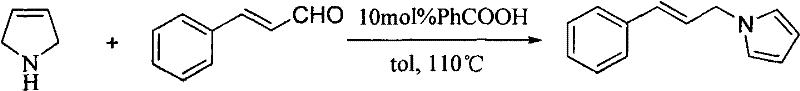Method for preparing N-(3'-aryl allyl)pyrrole derivatives
A technology of phenyl and compound, applied in the field of preparation of N-pyrrole derivatives, can solve the problems of harsh reaction conditions, high price, instability and the like, and achieves the effects of high yield, simple and easy operation, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of N-(3-phenylallyl)pyrrole:
[0033]
[0034] Cinnamaldehyde (0.66g, 0.63ml, 5mmol) and 4-acetoxy-L-proline (1.37g, 7.5mmol) were suspended in 10mL of redistilled N,N-dimethylformamide (DMF) , stirred and heated to 125 ° C for 4 h, the reaction solution was naturally cooled, the solvent was evaporated under reduced pressure, and silica gel column chromatography (eluent was petroleum ether / ethyl acetate = 10:1 (v / v)) to obtain N-benzene Pronyl-1H-pyrrole 0.65g, yellow liquid, yield: 71%;
[0035] 1 H NMR (400MHz, CDCl3) ppm 7.27 (5H, m), 6.68 (2H, s), 6.44 (1H, d, J=15.83Hz), 6.28 (1H, td, J=15.77, 6.10, 6.10Hz), 6.18(2H, s), 4.58(2H, d, J=6.00Hz);
[0036] 13 C NMR (101MHz, CDCl3) ppm 136.34, 132.60, 128.67, 127.96, 126.58, 125.57, 120.70, 108.49, 51.62;
[0037] MS, m / z(relative intensity): 183.1(69.1), 117.1(100), 184.1(7.44), 182.1(4.8), 143.1(2.6), 128.1(2.6), 118.1(7.9), 116.1(6.9), 115.1 (37.3).
Embodiment 2
[0039] Preparation of N-(3-phenylallyl)pyrrole:
[0040]
[0041] Except that the amount of 4-acetoxy-L-proline is changed from (1.37g, 7.5mmol) to (0.913g, 5mmol), other is the same as in Example 1, silica gel column chromatography (eluent is sherwood oil / Ethyl acetate=10:1 (v / v)) separated by silica gel column chromatography to obtain 0.50 g of N-phenylpropenyl-1H-pyrrole, yellow liquid, yield: 54.6%;
[0042] 1 H NMR (400MHz, CDCl3) ppm 7.27 (5H, m), 6.68 (2H, s), 6.44 (1H, d, J=15.83Hz), 6.28 (1H, td, J=15.77, 6.10, 6.10Hz), 6.18(2H, s), 4.58(2H, d, J=6.00Hz);
[0043] 13 C NMR (101MHz, CDCl3) ppm 136.34, 132.60, 128.67, 127.96, 126.58, 125.57, 120.70, 108.49, 51.62;
[0044] MS, m / z(relative intensity): 183.1(69.1), 117.1(100), 184.1(7.44), 182.1(4.8), 143.1(2.6), 128.1(2.6), 118.1(7.9), 116.1(6.9), 115.1 (37.3).
Embodiment 3
[0046] Preparation of N-(3-phenylallyl)pyrrole:
[0047]
[0048] Cinnamaldehyde (0.66g, 0.63ml, 5mmol) and 4-acetoxy-L-proline (1.37g, 7.5mmol) were suspended in 10mL[bmIm]BF 4 , stirred and heated to 125°C for 12 hours, the reaction solution was cooled naturally, poured into water after the reaction solution was naturally cooled, extracted with ethyl acetate, dried over anhydrous sodium sulfate, the filtrate was evaporated to remove the solvent under reduced pressure, silica gel column chromatography (elution The agent is petroleum ether / ethyl acetate=10:1 (v / v)) to obtain N-phenylpropenyl-1H-pyrrole 0.58g, yellow liquid, yield: 63.4%;
[0049] 1 H NMR (400MHz, CDCl3) ppm 7.27 (5H, m), 6.68 (2H, s), 6.44 (1H, d, J=15.83Hz), 6.28 (1H, td, J=15.77, 6.10, 6.10Hz), 6.18(2H, s), 4.58(2H, d, J=6.00Hz);
[0050] 13 C NMR (101MHz, CDCl3) ppm 136.34, 132.60, 128.67, 127.96, 126.58, 125.57, 120.70, 108.49, 51.62;
[0051] MS, m / z(relative intensity): 183.1(69.1), 117.1(100), 184...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com