Cloning, expression and application of a β-glucosidase gene
A glucosidase and gene technology, applied in the field of genetically engineered bacteria that secrete β-glucosidase, can solve the problems of unsuitable lactose decomposition, β-glucosidase cannot take into account both high temperature activity and low temperature activity, etc., and achieves safety The effect of strengthening, increasing security, and strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
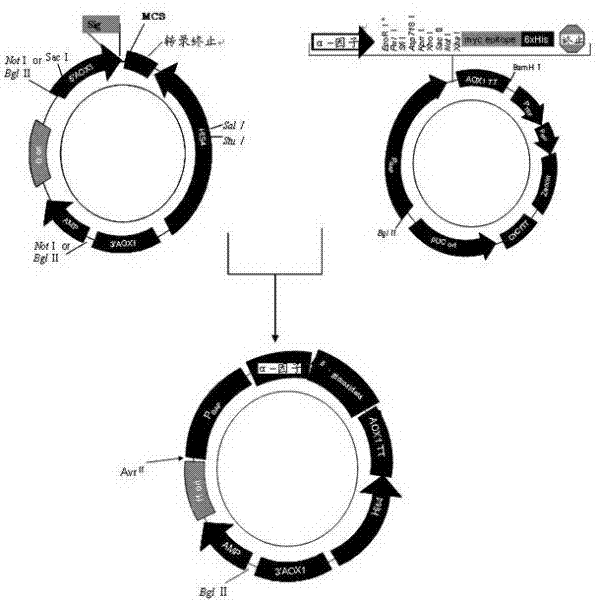
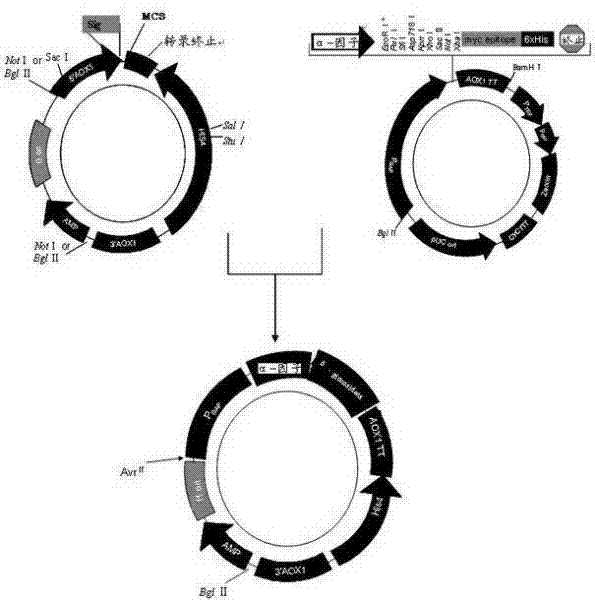
[0053] Embodiment 1: Construction of recombinant plasmid
[0054] Extract the total DNA template: take pyrococcus furiosus 20 mg of bacterial cells were added to DNA extraction solution (including 20 ml Tris-HCl pH8, 25 mM EDTA, 0.1 M NaCl, 0.5% SDS, 10 μg / ml proteinase K) to digest for 12 hours. Extract twice with phenol / chloroform (24:1 by volume) and once with chloroform. Separate the supernatant and add twice the volume of ethanol (100% concentration) to precipitate the DNA. The DNA was collected by centrifugation, dissolved in TE buffer, and PCR reaction was performed after the concentration was determined.
[0055] Construct the recombinant plasmid β-glucosidase / pCR2.1: According to the β-glucosidase gene sequence (Genebank pF0073), design two PCR primers:
[0056] They are: G001 5′-atgaagttcccaaaaaacttcatgtttgg-3′;
[0057] G002 5'-ctactttcttgtaacaaatttgaggtctgcg-3';
[0058] The gene was amplified by PCR, and then directly cloned into the PCR2.1-Top10 plasmid, a...
Embodiment 2
[0059] Example 2: Site-directed mutagenesis of recombinant plasmids
[0060] Refer to the online primer design software QuikChange?? Primer Design Program of Stratagene Company, and use the recombinant plasmid β-glucosidase / pCR2.1 as a template to design two mutation primers, respectively:
[0061] 371-F: 5′-ctaccaatgataatt gca gagaacggtatggccgatgcagca-3′;
[0062] 371-R: 5′-ggccataccgttctc tgc aattatcattggtagctcgatgg-3′;
[0063] The 371st amino acid of the recombinant plasmid β-glucosidase / pCR2.1 was subjected to site-directed mutation to prepare the recombinant mutant plasmid pGAPHa / β-glucosidase. Among them, TAC at positions 1111-1113 of the target gene was mutated into GCA, so that threonine at position 371 was mutated into leucine. The gene site-directed mutagenesis reaction system is shown in Table 1, and the PCR reaction parameters are shown in Table 2. .
[0064] After PCR, after hydrolysis with DpnI enzyme at 37°C for 1 hour, the mutant plasmid was transformed...
Embodiment 3
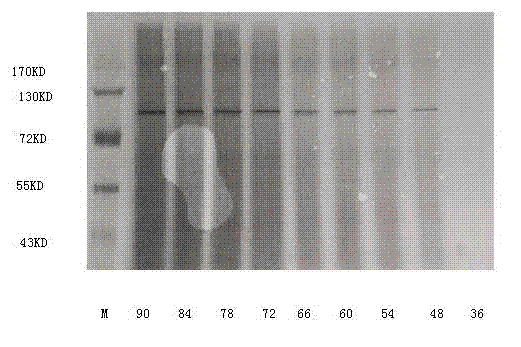
[0070] Example 3: Preparation of target DNA and electrotransformation of Pichia pastoris
[0071] Extract the plasmid pGAPHa / β-glucosidase, use AvrII / BglII for enzyme digestion and linearization, and then purify by agarose electrophoresis to obtain linear target DNA; prepare electrocompetent cells of Pichia pastoris GS115; mix 50ul of electrocompetent cells with 5ul (3 micrograms) of linearized DNA was mixed, transferred to a 0.4cm spacing electroporation cuvette, and electroporated at a voltage of 800V, and transformed into Pichia pastoris host strain GS115, which was constructed to express β-glucosidase yeast engineering bacteria.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com