Omeprazole enteric-coated capsules and preparation method thereof
A technology of omeprazole intestine and omeprazole, which is applied in the field of pharmaceutical preparations, can solve the problems of acid resistance and stability that need to be further improved, and achieve the effects of easy operation, good reproducibility and good acid resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
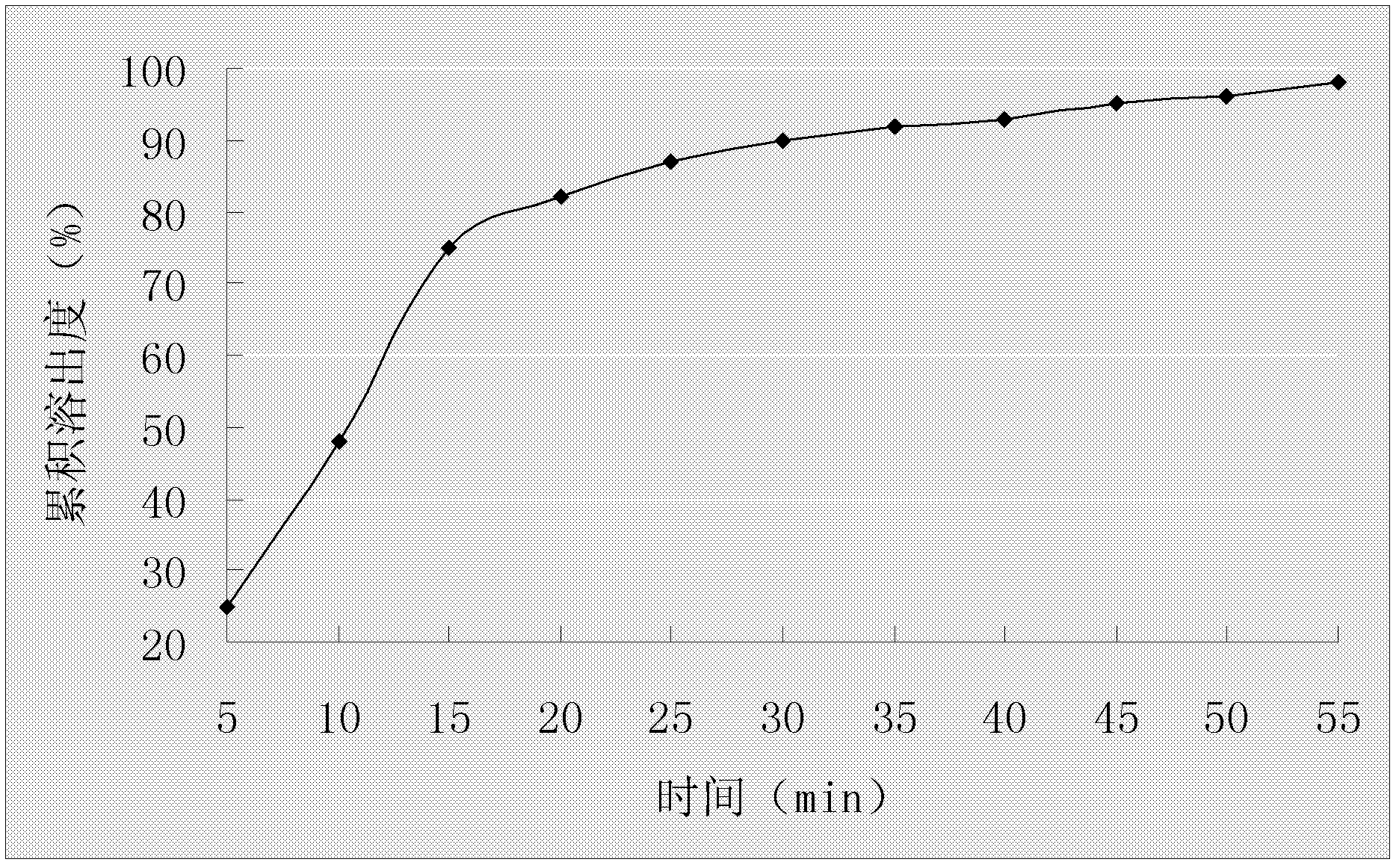
Embodiment 1
[0034] Embodiment 1 The preparation of omeprazole enteric-coated capsules
[0035] Prescription composition:
[0036]
[0037]
[0038] Preparation Process:
[0039] (1) After preparing a saturated aqueous solution of hydroxypropyl-β-cyclodextrin, pour it into a high-speed milk mixer at a speed of 3000r / min, stir at 35-38°C for 15min, add ammonium bicarbonate, and stir to dissolve;
[0040] (2) After dissolving omeprazole with ethanol, add it dropwise in a high-speed emulsion mixer, stirring at a rotating speed of 3000r / min for 55min;
[0041] (3) The resulting mixed solution is dried by spray drying, and the omeprazole cyclodextrin inclusion compound is collected, wherein the spray drying parameters are: inlet temperature 40°C-45°C, spray speed 5ml / min, and spray pressure of 5 bar;
[0042] (4) Add omeprazole inclusion compound and hydroxypropyl methylcellulose phthalate to 50% ethanol solution, stir and dissolve at 40-50°C, keep 45-50°C for rotary evaporation under ...
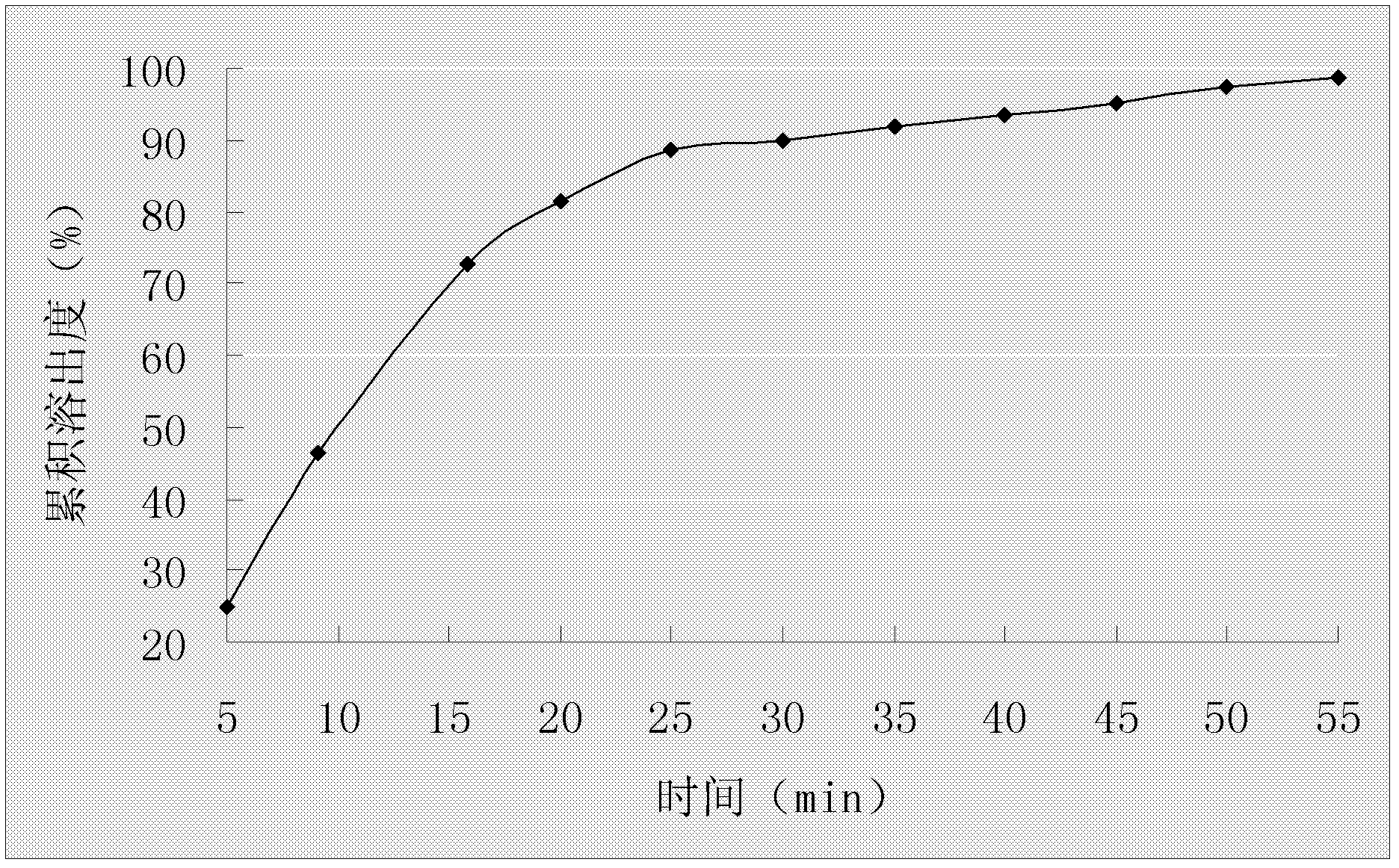
Embodiment 2
[0045] Example 2 Preparation of Omeprazole Enteric-Coated Capsules
[0046] Prescription composition:
[0047]
[0048] Preparation Process:
[0049] (1) After preparing a saturated aqueous solution of hydroxypropyl-β-cyclodextrin, pour it into a high-speed milk mixer at a speed of 2800r / min, stir at 35-38°C for 18min, add ammonium bicarbonate, and stir to dissolve;
[0050] (2) Omeprazole is dissolved in ethanol and then added dropwise to a high-speed emulsion mixer, stirring at a speed of 2500r / min for 50min;
[0051] (3) The resulting mixed solution is dried by spray drying, and the inclusion compound of omeprazole cyclodextrin is collected, wherein the spray drying parameters are: inlet temperature 40°C-45°C, spray speed 8ml / min, and spray pressure of 3 bar;
[0052] (4) Add omeprazole inclusion compound and hydroxypropyl methylcellulose phthalate to 50% ethanol solution, stir and dissolve at 40-50°C, keep 40-45°C for rotary evaporation under reduced pressure solven...
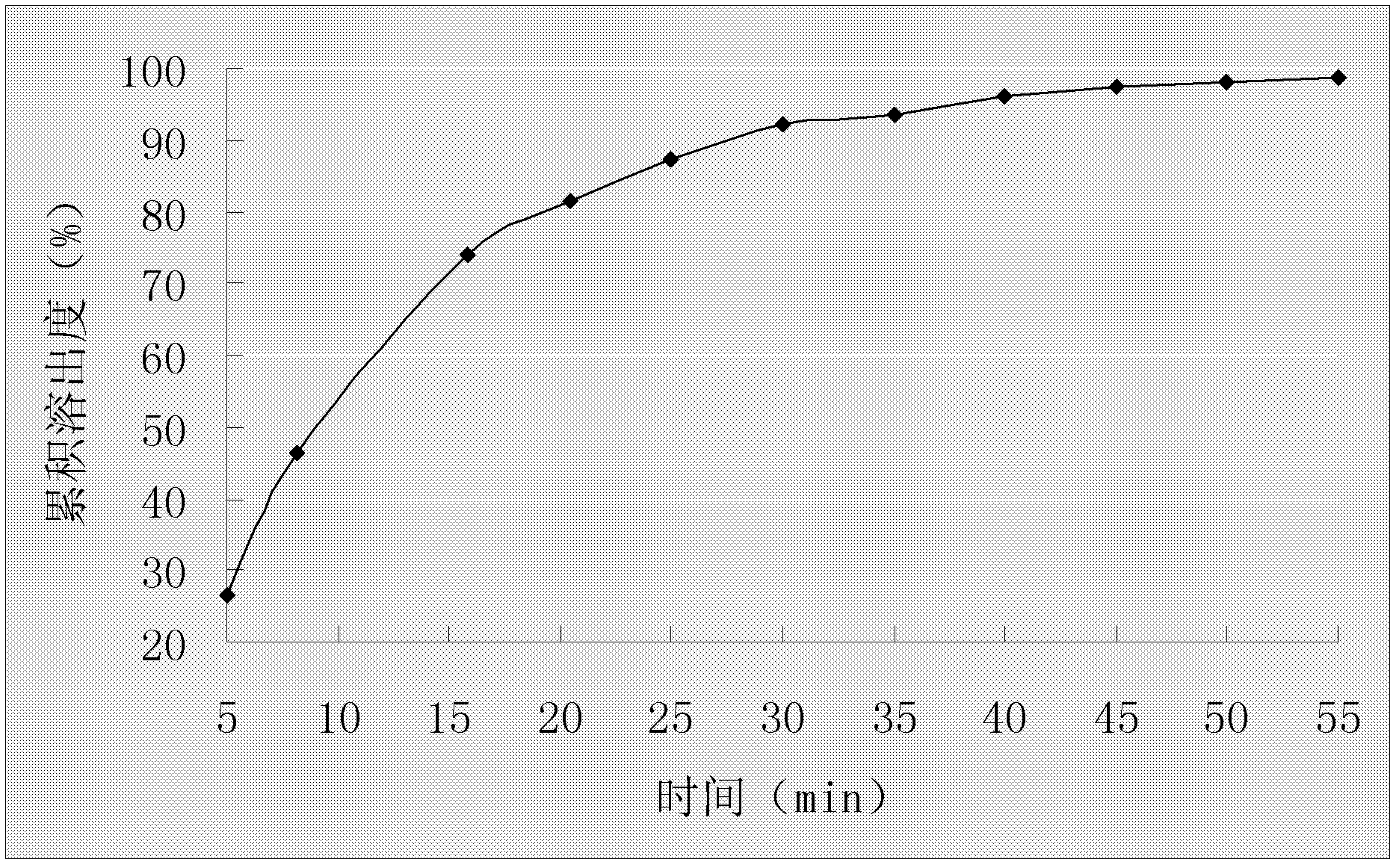
Embodiment 3
[0055] Example 3 Preparation of Omeprazole Enteric-Coated Capsules
[0056] Prescription composition:
[0057]
[0058] Preparation Process:
[0059] (1) After preparing a saturated aqueous solution of hydroxypropyl-β-cyclodextrin, pour it into a high-speed milk mixer at a speed of 3000r / min, stir at 35-38°C for 15min, add ammonium bicarbonate, and stir to dissolve;
[0060] (2) Omeprazole is dissolved in ethanol and then added dropwise to a high-speed emulsion mixer, stirring at a speed of 3000r / min for 50min;
[0061] (3) The resulting mixed solution is dried by spray drying, and the inclusion compound of omeprazole cyclodextrin is collected, wherein the spray drying parameters are: inlet temperature 40°C-45°C, spray speed 5ml / min, and spray pressure of 2 bar;
[0062] (4) Add omeprazole clathrate and hydroxypropyl methylcellulose phthalate to 50% ethanol solution, stir and dissolve at 40-50°C, keep 42-45°C for rotary evaporation under reduced pressure solvent to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com