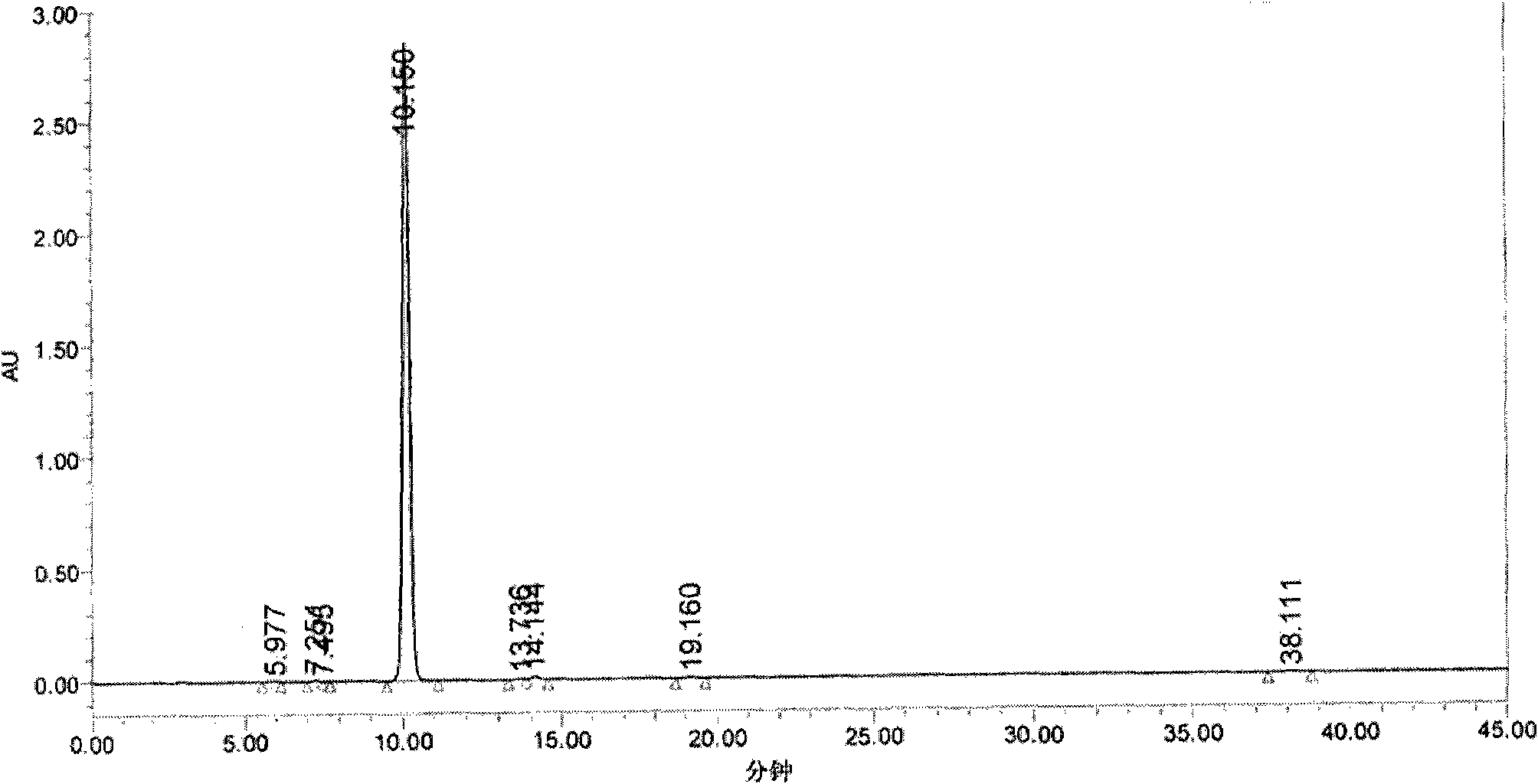
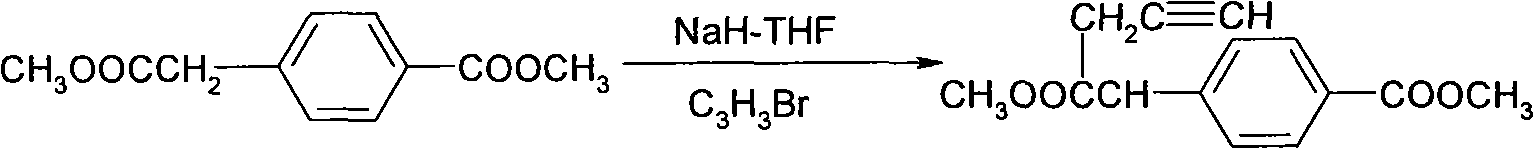Preparation method of 2-aryl-pentyne-4-olic acid ester compounds
A compound and alkyl technology, applied in the field of preparation of 2-aryl-pent-4-ynoic acid ester compounds, can solve the problems of harsh reaction conditions, unsuitable for large-scale industrial production, and difficult to separate, and achieves easy industrialization production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of 2-(4-(ethoxycarbonyl)phenyl)-2-(prop-2-ynyl)-malonate (III)
[0051] Dissolve 2-(4-(ethoxycarbonyl)phenyl)-malonic acid diethyl (IV) (12.3g, 40mmol) and 3-bromopropyne (5.72g, 48mmol) in acetone (100mL) Add potassium carbonate (22.1g, 160mmol), heat to reflux, stir for 2 hours, cool to room temperature, add a large amount of water to dilute, adjust pH to 3-4 with 2N dilute hydrochloric acid, extract with ethyl acetate (300mL*3), and combine The organic phase was washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, the filtrate was evaporated to dryness, 200-300 mesh silica gel column chromatography (petroleum ether: ethyl acetate = 20:1), and the solvent was evaporated to obtain a yellow oil 物9.7g.
Embodiment 2
[0053] Preparation of 2-(4-(methoxycarbonyl)phenyl)-2-(prop-2-ynyl)-malononitrile (III)
[0054] Dissolve 2-(4-(methoxycarbonyl)phenyl)-malononitrile (IV) (10.0g, 50mmol) and 3-bromopropyne (8.92g, 75mmol) in acetone (120mL), add carbonic acid Potassium (17.3g, 125mmol), heat and stir to reflux, after 2 hours, cool to room temperature, add a large amount of water to dilute, adjust the pH to 3-4 with 2N dilute hydrochloric acid, extract with ethyl acetate (300mL*3), combine the organic phases, Wash with water, saturated brine, dry with anhydrous magnesium sulfate, filter, evaporate the filtrate, 200-300 mesh silica gel column chromatography (petroleum ether: ethyl acetate = 20:1), evaporate the solvent to obtain 8.9 g of yellow solid.
Embodiment 3
[0056] Preparation of 2-[(4-carboxy)phenyl]-pent-4-ynoic acid (II)
[0057] The 2-(4-(ethoxycarbonyl)phenyl)-2-(prop-2-ynyl)-malonic acid diethyl (III) (9.7g, 28.0mmol) obtained in Example 1 was dissolved in In methanol (30mL), add potassium hydroxide (15.8g, 280mmol) aqueous solution (30mL), warm to reflux, stir for 2 hours, cool to room temperature, add water to dilute, evaporate methanol under reduced pressure, ethyl acetate (100mL*3 ) Extract, combine the organic phases, wash with water, wash with saturated brine, dry with anhydrous sodium sulfate, filter, evaporate the filtrate, and recrystallize (petroleum ether: ethyl acetate = 1:1) to obtain 5.8 g of white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com