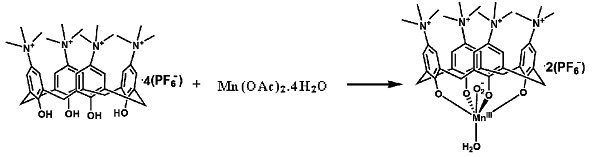A kind of calixarene derivative and its metal complex as well as their preparation method and application
A technology of metal complexes and derivatives, which is applied in the preparation of organic compounds, preparation of aminohydroxyl compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problem that aromatics have not been found and reported, and achieve high conversion rate , mild reaction conditions, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
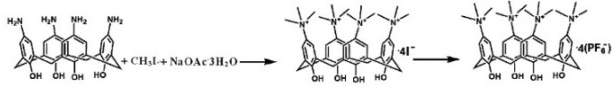
[0047] Embodiment one: preparation [H 4 L](PF 6 ) 4 (where H 4 L=5,11,17,23-tetrakis (trimethylquaternary ammonium salt) calix [4] arene) method comprises the following steps:
[0048] [1] Preparation of p-benzoic acid diazonium chloride salt solution:
[0049] Dissolve 1.37g (10mmol) of p-aminobenzoic acid in 10mL of 5% (mass fraction) sodium hydroxide solution, add 0.69g (10mmol) of sodium nitrite after cooling to room temperature, and continue stirring for 30 minutes, then put the above solution in Slowly add dropwise to the mixture of 3.67mL concentrated hydrochloric acid and 2.5mL distilled water while stirring, keep the temperature at 0-5°C, stir for 30 minutes after the dropwise addition is completed, a large amount of white precipitate is found, dissolve after adding 4mL distilled water .
[0050] [2] Preparation of calix[4]arenes
[0051] Calix[4]arene Reference Synthesis: Gutsche, C.D.; Levine, J.A.; Sujeeth, P.K. J. Org. Chem .1985, 50 ,5802.
[0052] [3] ...
Embodiment 2
[0073] [1] see image 3 , to prepare the calix[4]arene Mn(III) superoxide complex [Mn(III)L(O 2 )(H 2 O)](PF 6 ) 2
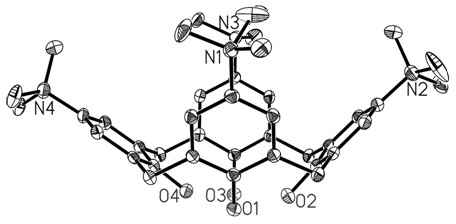
[0074] 2mlMn(OAc) 2 4H 2 O (98mg, 0.4mmol) methanol solution was slowly added dropwise to 6ml [H 4 L](PF 6 ) 4 (where H 4 L = 5,11,17,23-tetra(trimethylquaternary ammonium salt) calix[4]arene) (123mg, 0.1mmol) in acetonitrile solution, the mixed solution was stirred in an open air system for 3 hours, filtered, Diethyl ether was added to the filtrate to diffuse at room temperature for 1 week, and dark purple rhombic crystals appeared, filtered, washed with ether, and dried to obtain the target product [Mn(III)L(O 2 )(H 2 O)](PF 6 ) 2 , the quaternary ammonium-containing calix [4] arene metal complex [Mn (III) L (O 2 )(H 2 O)](PF 6 ) 2 The schematic diagram of the cation structure is attached Figure 4 shown.
[0075] [2] The product was characterized by infrared and X-ray single crystal diffraction.
[0076] The specific results are as follows:...
Embodiment 3
[0083] At 20°C, olefin (0.88 mmol), isobutyraldehyde (1.76 mmol), catalyst [Mn(III)L(O 2 )(H 2 O)](PF 6 ) 2 (1.76 × 10 -3 mmol) of 2 mL MeCN solution was added to a 10 mL round bottom flask. After the mixture was stirred for 5 hours under the condition of bubbling oxygen, the bubbling of oxygen was stopped and filtered. Various components in the mixed solution were analyzed by gas chromatography-mass chromatography (GC-MS) test and internal standard comparison. The epoxidized products were further identified by gas chromatography (GC) retention times and internal standards.
[0084] The selection of the olefins is shown in the substrate column in the table below, and the products obtained and the corresponding selectivity are shown in the table below:
[0085]
[0086] As can be seen from the above table: it can be seen that the calix [4] arene Mn (III) superoxide complex [Mn (III) L (O 2 )(H 2 O)](PF 6 ) 2 It can be used to catalyze the epoxidation reaction of o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com