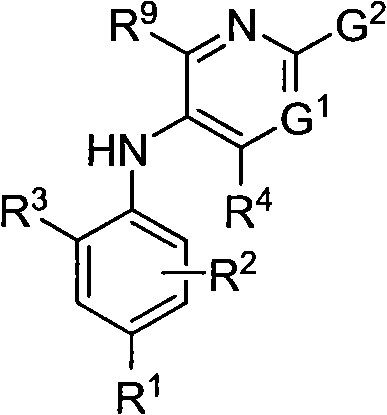
Combinations that include methotrexate and a dihydroorotate dehydrogenase inhibitor
A technology of dihydroorotic acid and hydrogen atoms, which can be used in drug combinations, anti-inflammatory agents, anti-toxic agents, etc., can solve problems such as hindering the development of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0347] Example 1 - Determination of Inhibition of Human DHODH Activity
[0348] DHODH activity and its inhibition were studied with 2,6-didichlorophenol-indophenol (DCIP) using a chromogen reduction assay. Combines substrate oxidation (dihydroorotate, L-DHO) and co-substrate reduction (coenzyme Q, CoQ) with chromogen reduction, thus enzymatic activity results in chromogen absorbance at 600 nm Loss.
[0349] Enzyme extracts (8 microliters, approximately 1.5 micrograms of human protein) were incubated in 96-well plates. Assay mix (200 μl) contained 200 μM CoQD, 100 μM L-DHO, 120 μM DCIP, and 2 μl Test compound. Compounds were dissolved in DMSO such that the stock concentration was 1 mM and tested at different concentrations ranging from 10 μM to 1 pM to calculate the IC 50 (inhibitor concentration required for 50% inhibition).
[0350] The reaction was initiated by the addition of enzyme, followed by incubation for 10 minutes at room temperature, followed by measurement of...
example 2
[0355] Example 2 - Reduced liver toxicity
[0356] Acute hepatotoxicity assays were performed on Swiss mice. Animals were administered either vehicle, or 100 mg / kg teriflunomide, or a compound of the invention (from the list shown above) alone by intraperitoneal route. After 24 hours, the animals were sacrificed and the levels of the liver markers AST (aspartate aminotransferase), ALT (alanine aminotransferase) and BIL (total bilirubin) in plasma were determined.
[0357] Table 2: After administration of 100 mg / kg compound, 100 mg / kg teriflunomide or vehicle Plasma levels (IU: International Units) of liver markers in mice after administration.
[0358] Compound number
[0359] As is clear from Table 2, compared to vehicle-treated mice, teriflunomide-treated mice showed a significant increase in three liver markers, clearly indicating high hepatotoxicity, whereas DHODH of the present invention Inhibitors did not cause significant increases in any of the parame...
example 3
[0360] Example 3: Determination of the efficacy of the combination product of the invention in adjuvant-induced arthritis.
[0361] The effect of DHODH inhibitor compounds in combination with methotrexate (0.05 mg / kg per day) was tested in a rat adjuvant-induced arthritis model (AIA) on animals with established disease (cure gender program). Briefly, Complete Freund's Adjuvant (CFA) was injected into the left hind paw of Wistar rats, and 10 days later, the organ plethysmometer (plethysmometer) was used to measure the Swelling condition. Rats exhibiting similar degrees of inflammation in both paws were randomized into treatment groups (n=7 per group). Compounds were administered orally once daily for 10 days and paw volume was measured every other day until day 21.
[0362] Table 3. Compound 20 (10 mg / kg per day), methotrexate (0.05 mg per day / kg) and their combination on the inhibition of paw inflammation in arthritic rats.
[0363] Results are expressed as percent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com