A polymer microsphere
A polymer and microsphere technology, which is applied in the preparation of microspheres, microcapsule preparations, etc., can solve the problems of low epoxy group content, poor mechanical properties, and reduced mechanical properties, and achieve high mechanical strength, strong impact resistance, The effect of uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] This embodiment provides a method for preparing polymer microspheres, comprising the following steps:
[0026] 1. Microsphere surface bonds and initiator molecules
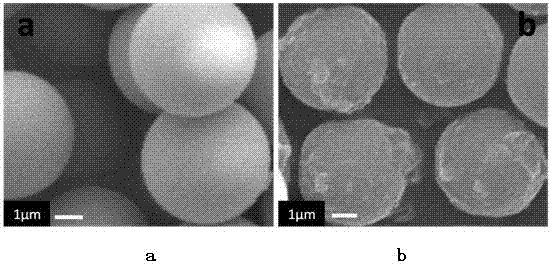
[0027] Monodisperse polystyrene-divinylphenyl spheres with a cross-linking degree of 80% and a particle size of 10 microns and α-haloacyl halides, catalysts (the molar ratio of catalyst to α-haloacyl halides is 1:1) mixed with an appropriate amount of solvent, stirred at 40°C for 24 hours to obtain microspheres of surface bonds and initiator molecules.
[0028] In this example, the α-haloacyl halide can be selected from α-bromobutyryl bromide, α-bromoisobutyryl bromide, α-bromopropionyl bromide, α-chlorobutyryl bromide, α-chloro One of isobutyryl bromide or α-chloropropionyl bromide. Catalyst can choose FeCl 3 , SnCl 4 , BF 3 , ZnCl 2 Or one of AlCl. The solvent can be selected from one of dichloromethane, 1,2-dichloroethane, carbon disulfide or nitrobenzene.
[0029] 2. Grafting polyglycidyl methacry...
Embodiment 2
[0042] This embodiment provides a method for preparing polymer microspheres, comprising the following steps:
[0043] 1. Using Friedel-Crafts acylation reaction to prepare microspheres with surface bonds and initiator molecules
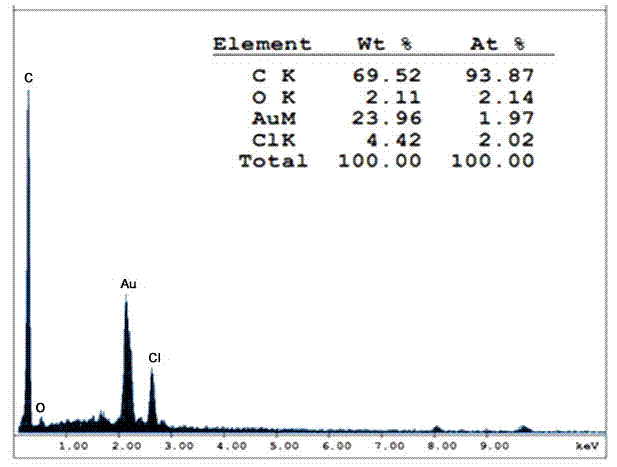
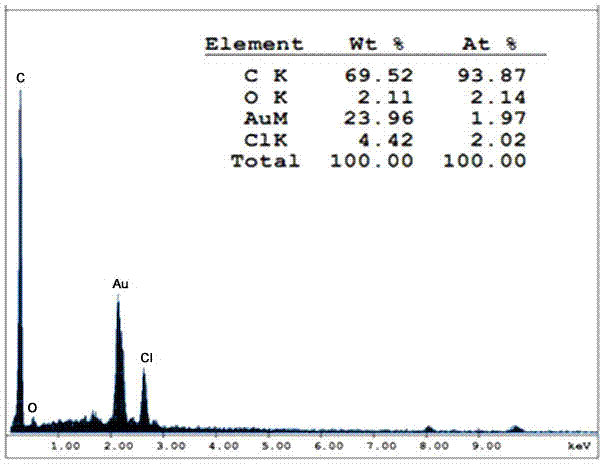
[0044] In a dry 250ml two-necked flask, add 8g of raw material microspheres, the microspheres are monodisperse polystyrene-divinylbenzene microspheres with a crosslinking degree of 60% and a particle size of 20 microns, and then add solvent 1,2 - 46ml of dichloroethane, slowly add 21.76g of anhydrous aluminum trichloride under magnetic stirring, then dropwise add 7.2ml of acylating reagent chloropropionyl chloride, dropwise, and react at a temperature of 30°C for 24 hours. Add 4 ml of concentrated hydrochloric acid and 144 g of crushed ice to the reacted system, stir for 20 minutes, then filter with suction, and wash with water, ethanol, and acetone several times respectively. The obtained microspheres were subjected to surface elemental analysis, se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com