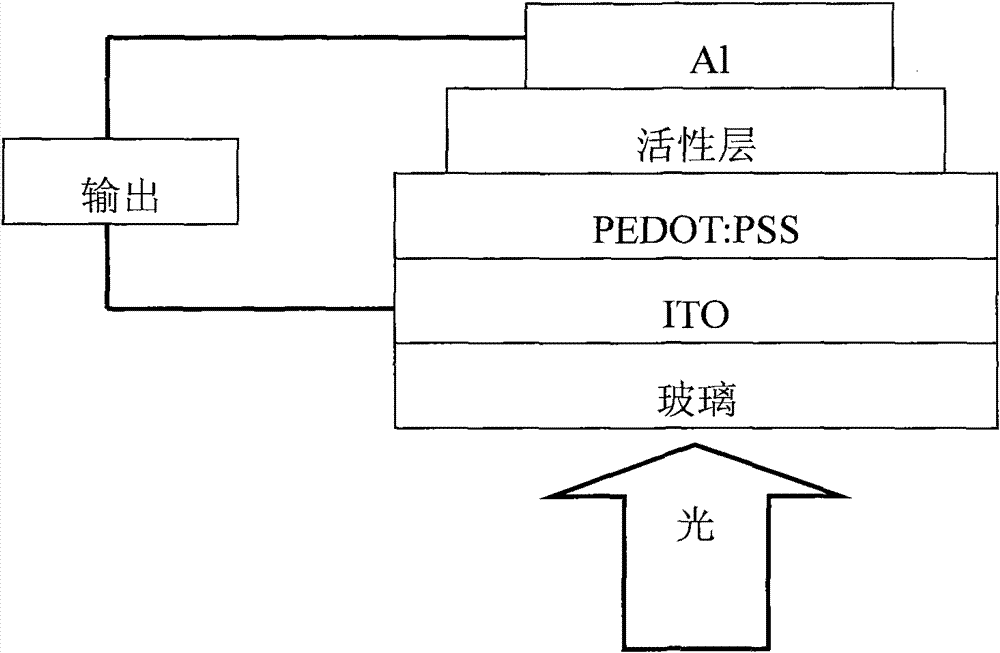
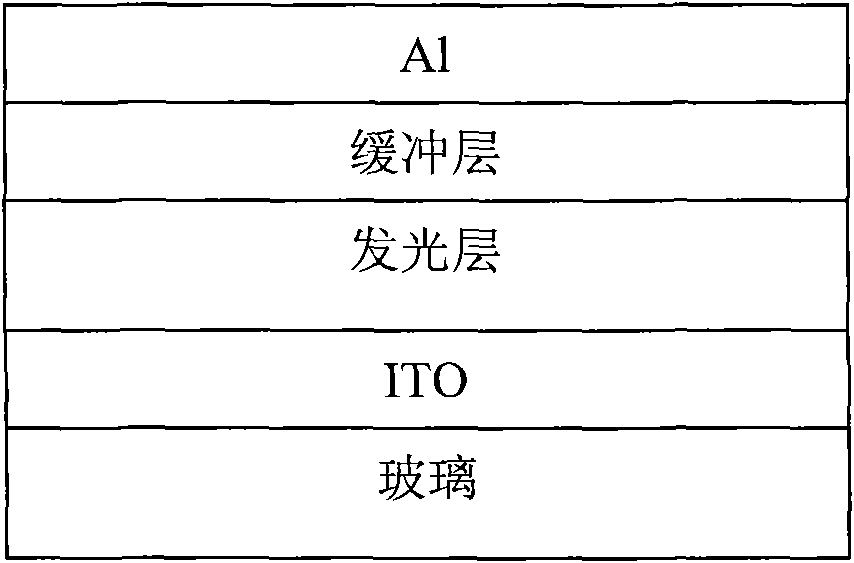
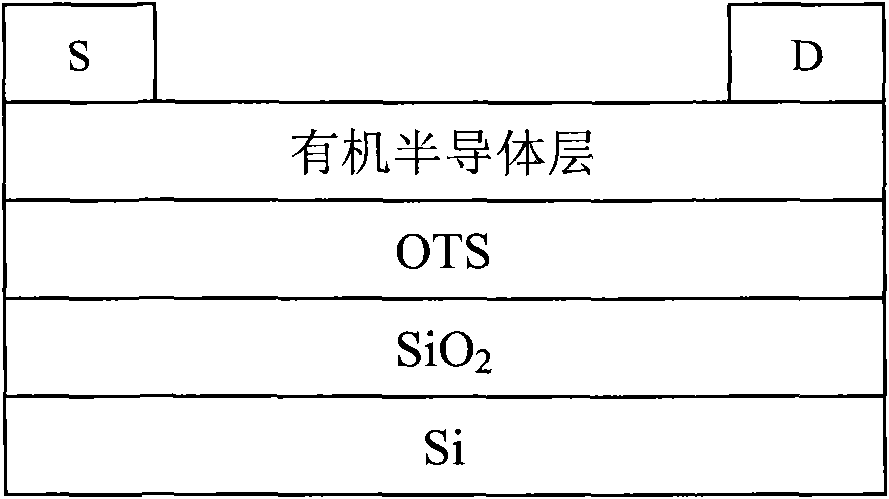
Copolymer material containing carbazole porphyrin-triphenylamine and preparation method and application thereof
A technology of carbazole porphyrin and triphenylamine, which is applied in the field of carbazole porphyrin-triphenylamine-containing copolymer material and its preparation, and can solve the problem of low conversion efficiency of inorganic solar cells, mismatched spectral response, and low carrier electrodes. Collection efficiency and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This embodiment discloses a 10,20-di(9-octylcarbazole) porphyrin-triphenylamine copolymer material (n=52) with the following structure:
[0050]
[0051] The preparation steps of the above-mentioned organic semiconductor material are as follows:
[0052] One, the synthesis of 4,4'-two (4,4,5,5-tetramethyl-1,3,2-dioxaborolane) triphenylamine:
[0053]
[0054] Under the protection of nitrogen, add p-4,4'-dibromotriphenylamine (8.0g, 0.02mol) into the three-necked flask, then add 150ml of tetrahydrofuran solvent, and slowly inject n-butyl Lithium (16.8mL, 2.5M, 0.04mol), continue to stir the reaction for 2h, inject 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2 - Dioxaborolane (8.7mL, 0.04mol), stirred at room temperature for 6h-16h. Saturated aqueous sodium chloride (30ml) was added to terminate the reaction, extracted with chloroform, dried over anhydrous sodium sulfate, and filtered, the filtrate was collected and the solvent was evaporated off. Finally, the crude pro...
Embodiment 2
[0065] This embodiment discloses a 10-(9-methylcarbazole)-20-(9-dodecylcarbazole) porphyrin triphenylamine copolymer material (n=9) with the following structure:
[0066]
[0067] The preparation steps of the above-mentioned organic semiconductor material are as follows:
[0068] One, the synthesis of 4,4'-two (4,4,5,5-tetramethyl-1,3,2-dioxaborolane) triphenylamine:
[0069] Its preparation sees embodiment 1 for details.
[0070] Two, the synthesis of 5-(9-methylcarbazole)-15-(9-docosylcarbazole) porphyrin:
[0071]
[0072] Set up an anhydrous and oxygen-free device, weigh the intermediate 2-aldehyde-9-methylcarbazole (0.21g, 1mmol), 2-aldehyde-9-docosylcarbazole (0.65g, 1mmol), di Pyrromethane (0.30g, 2mmol), dissolved in 250ml of dichloromethane, blown nitrogen for 30min, added 2ml of trifluoroacetic acid into the syringe, stirred at 100°C for 1h, then added dichlorodicyanobenzoquinone (DDQ) (1.82g , 8mmol), continue to stir at room temperature for 30min, then add...
Embodiment 3
[0080] This embodiment discloses a 10-carbazole-20-(9-hexadecylcarbazole) porphyrin triphenylamine copolymer material (n=100) with the following structure:
[0081]
[0082] The preparation steps of the above-mentioned organic semiconductor material are as follows:
[0083]One, the synthesis of 4,4'-two (4,4,5,5-tetramethyl-1,3,2-dioxaborolane) triphenylamine:
[0084] Its preparation sees embodiment 1 for details.
[0085] Two, the synthesis of 5-carbazole-15-(9-hexadecylcarbazole) porphyrin:
[0086]
[0087] Set up an anhydrous and oxygen-free device, weigh intermediate 2-aldehyde carbazole (0.20g, 1mmol), 2-aldehyde-9-hexadecylcarbazole (0.42g, 1mmol), dipyrromethane (0.30g, 2mmol ), dissolved in 300ml of dichloromethane, fed nitrogen for 30min, added 2ml of trifluoroacetic acid into a syringe, stirred at room temperature for 3h, then added dichlorodicyanobenzoquinone (DDQ) (1.82g, 8mmol), and continued to Stir for 30min, then add 2ml triethylamine to quench the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com