Electrode used for vanadium redox flow battery and preparation method for electrode
An all-vanadium redox flow battery and electrode technology, which is applied to battery electrodes, circuits, electrical components, etc., can solve the problems of low voltage efficiency and achieve the effects of improving voltage efficiency, good electrochemical activity, and obvious effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of the present invention comprises:
[0026] Pre-surface-treat the metal foil / mesh;
[0027] Mixing the nano-carbon-based electrode material with the conductive agent and the binder in a weight ratio of 90:1-5:1-5;
[0028] The electrode material is deposited on the surface of the current collector to obtain an electrode for an all-vanadium redox flow battery.
[0029] In the present invention, the nano-carbon-based electrode material is one or more of graphene oxide, reduced graphene, carbide, carbon nitride or carbon / metal nitride composite materials; the conductive agent can be acetylene black, Super P, One or more of graphitized carbon fibers and vapor-grown carbon fibers; the binder is polyvinylidene fluoride PVDF, polytetrafluoroethylene PTFE, carboxylated styrene-butadiene latex SBR, sodium carboxymethylcellulose CMC, Nafion solution, etc.
[0030] In the preparation method of the present invention, the deposition method includes electros...
Embodiment 1
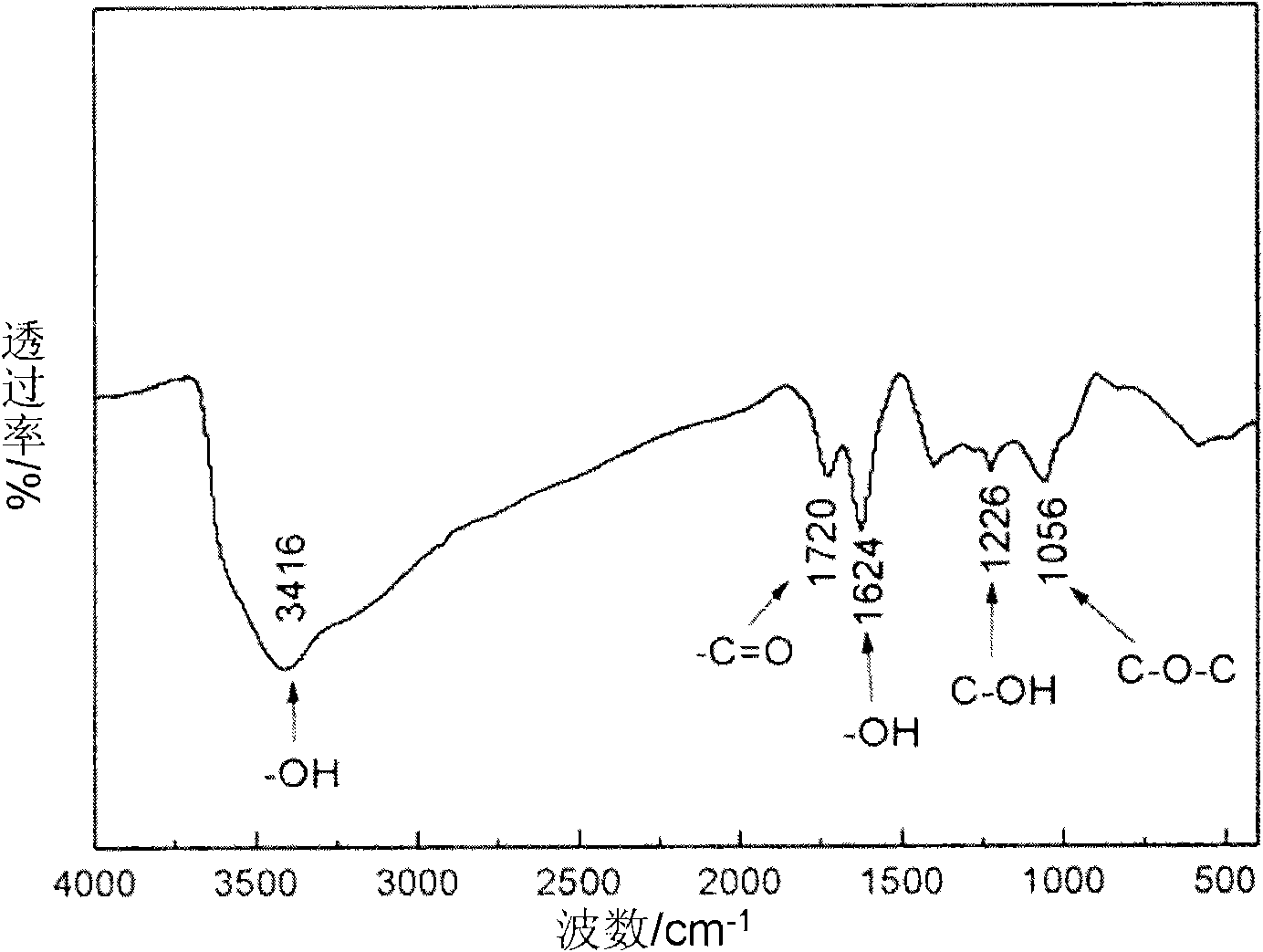
[0033] Get 23ml of 98wt% concentrated sulfuric acid and place it in a reaction vessel, keep the temperature at about 0°C with an ice-water bath, mix 1g of commercially available artificial graphite and 1NaNO 3 Add sulfuric acid and stir vigorously for 10min; slowly add 3g KMnO while stirring 4 , while controlling the reaction temperature below 20°C, remove the ice-water bath after the addition; continue to stir the above mixture in a 35°C water bath for 30 minutes, slowly add 46ml of water to raise the temperature to 98°C and keep it for 10 minutes; use warm water at about 50°C Dilute to 140ml, add H 2 o 2 (5wt%), filtered while hot, washed twice with 5wt% hydrochloric acid, and then washed with deionized water until no SO 4 2- Existence; dry the obtained material in a vacuum oven at 50°C for 24 hours, grind to obtain graphene oxide, and the measured specific surface area is 42.7m 2 / g.
[0034] Mix CMC (sodium carboxymethyl cellulose) and water to prepare a 1-3% solution...
Embodiment 2
[0037] The graphene oxide obtained in Example 1 was placed in a tube furnace and heat-treated at 120° C. for 12 hours at a heating rate of 5° C. min to obtain an electrode material. The measured specific surface area was 56.1 m 2 / g.
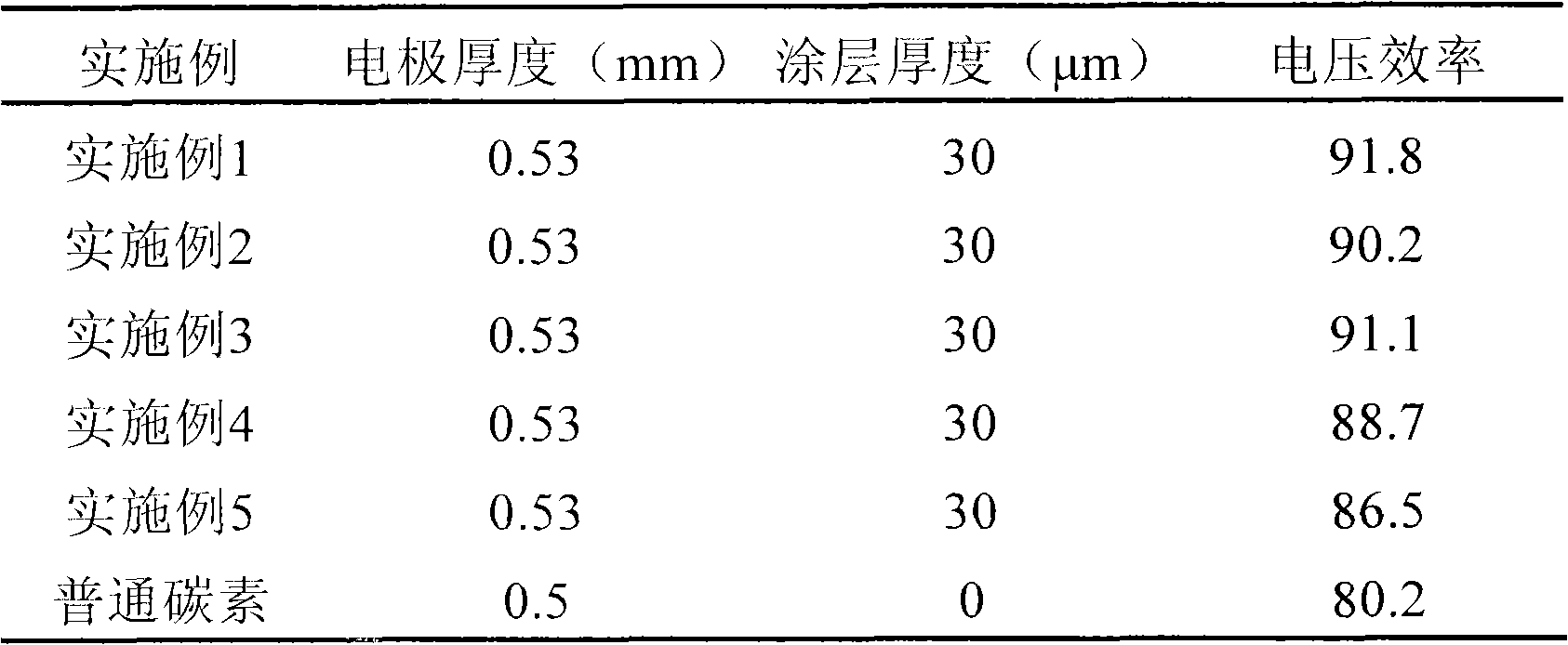
[0038] Except that the vacuum drying temperature of the electrode was changed to 120° C. for 24 hours, the preparation method was the same as in Example 1. The thickness of the electrode material was measured with a micrometer to be 30 μm. According to the ICP emission spectrum test result, the electrode material accounted for 7wt% of the entire electrode. The battery performance test method is the same as in Example 1, and the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Sheet thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com