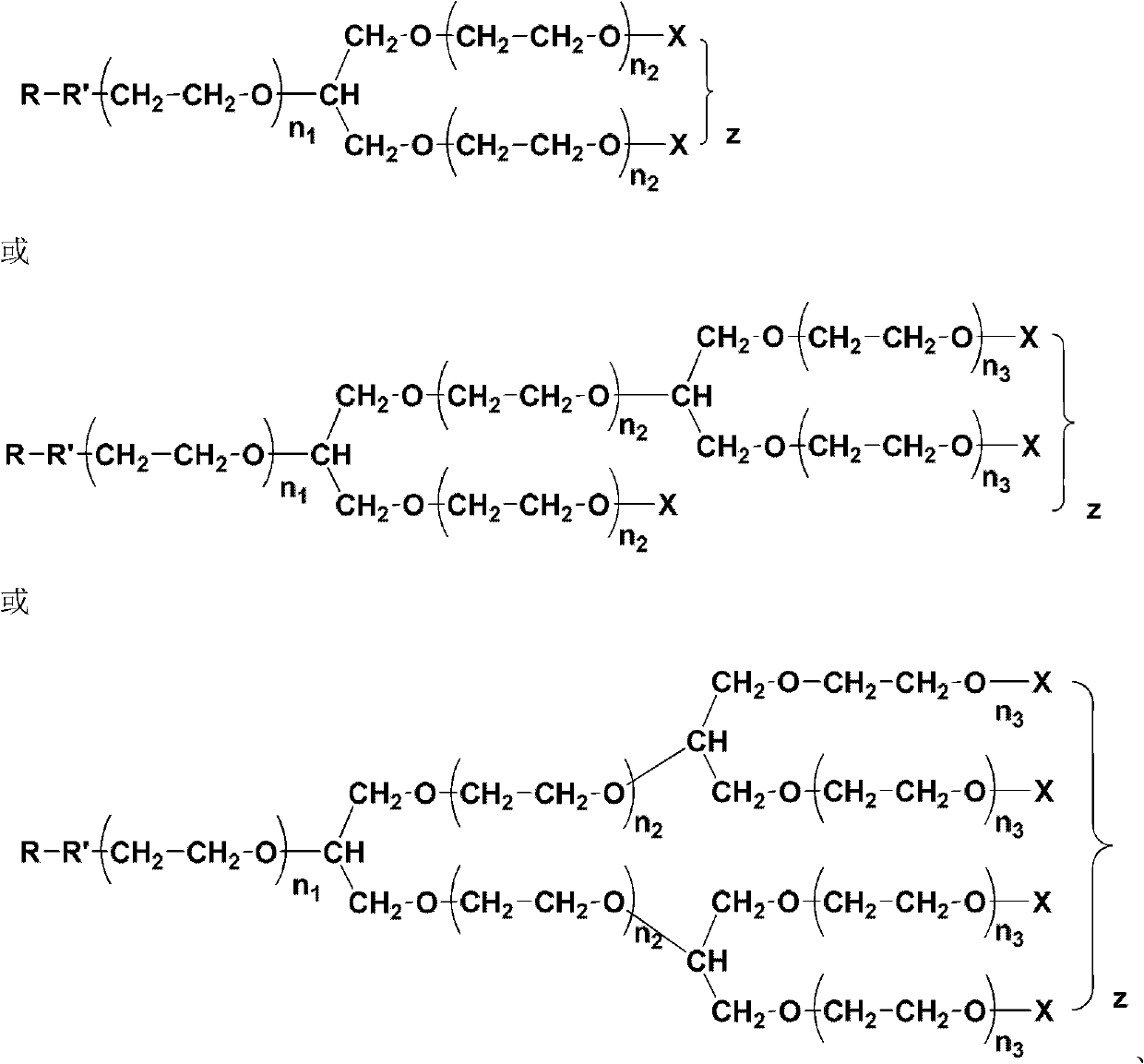
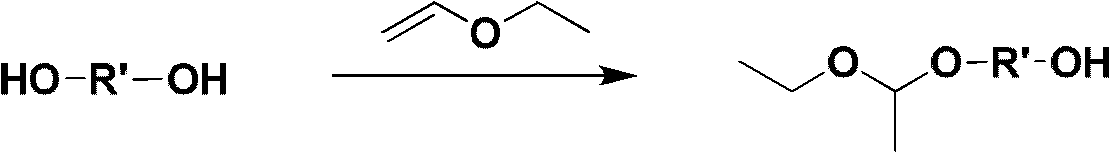
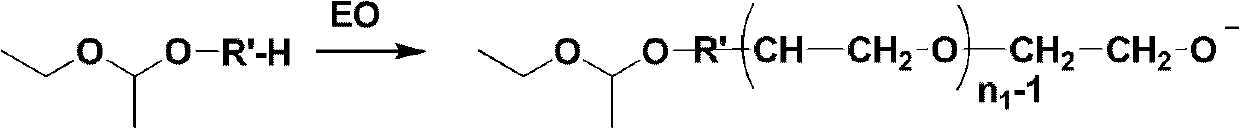
Mono-functional group-containing multilevel branched polyethylene glycol and its synthesis method
A polyethylene glycol and single-function technology, applied in the field of multi-level branched polyethylene glycol and its synthesis, can solve the problems of low yield, easy hydrolysis and fracture, affecting the use of reagents, etc., and achieve high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1.1 Preparation of initiator
[0049] At 0°C, 82.3g of ethylene glycol, 100.4g of vinyl ether and 85mL of tetrahydrofuran as a reaction solvent were successively added to a 500mL dry and clean round-bottomed flask, and then 2.43g of p-toluenesulfonic acid was added in batches as a catalyst. After reacting for 3-5 hours and stirring overnight, a saturated sodium carbonate aqueous solution was added dropwise until pH=7. Then it was dried with anhydrous magnesium sulfate, filtered, and unreacted vinyl ethyl ether and tetrahydrofuran were removed by rotary evaporation at 75°C. The product was purified by column chromatography with diethyl ether as eluent. After the diethyl ether is removed by rotary evaporation, diol derivatives (initiators) whose two ends are respectively protected hydroxyl groups and unprotected hydroxyl groups are obtained.
[0050]
[0051] The proton spectrum data of initiator are as follows:
[0052] 1 H NMR (CDCl 3 )δ (ppm): 1.20 (CH 3 CH 2...
Embodiment 2
[0070] R is OH, X is CH 3 Preparation of Tertiary Branched Polyethylene Glycol:
[0071] In a dry and clean 50mL round-bottomed flask, add 1g of the iodomethane-terminated polymer obtained in Example 1, 20mL of tetrahydrofuran and 0.5mL of concentrated hydrochloric acid, spin dry tetrahydrofuran and concentrated hydrochloric acid after 15 minutes of reaction, and obtain R as OH, X is CH 3 branched polyethylene glycol. R is OH, X is CH 3 The hydrogen spectrum data of the branched polyethylene glycol is as follows:
[0072] 1 H NMR (CDCl 3 )δ (ppm): 3.35 (CH 3 O-), 3.40-3.80 (-CH 2 CH 2 O-, glycidol).
Embodiment 3
[0074] R is for OCH 2 CH 2 CN, X is CH 3 Preparation of Tertiary Branched Polyethylene Glycol:
[0075] In a dry and clean 50mL round-bottomed flask, add 1g of R as OH and X as CH prepared in Example 2 3 Branched polyethylene glycol, 20mL dioxane and 0.2g potassium hydroxide, slowly add 1mL acrylonitrile dropwise at 0°C. After continuing to react at room temperature for 8 hours, dioxane was removed by rotary evaporation, the product was dissolved in chloroform and washed with water three times, and the chloroform phase was dried with anhydrous magnesium sulfate, filtered, concentrated, and precipitated to obtain R as OCH 2 CH 2 CN, X is CH 3 branched polyethylene glycol. R is for OCH 2 CH 2 CN, X is CH 3 The hydrogen spectrum data of the branched polyethylene glycol is as follows:
[0076] 1 H NMR (CDCl 3 )δ (ppm): 2.40 (-CH 2 CN), 3.35 (CH 3 O-), 3.40-3.80 (-CH 2 CH 2 O-, glycidol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com