Copolymer containing thieno pyrroledione units, preparation method, and application thereof
A technology of thienopyrrole diketone and copolymer, which is applied in the field of copolymers, can solve the problems of low photoelectric conversion efficiency, achieve the effects of improving photoelectric conversion efficiency, improving stability, and reducing energy bandgap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
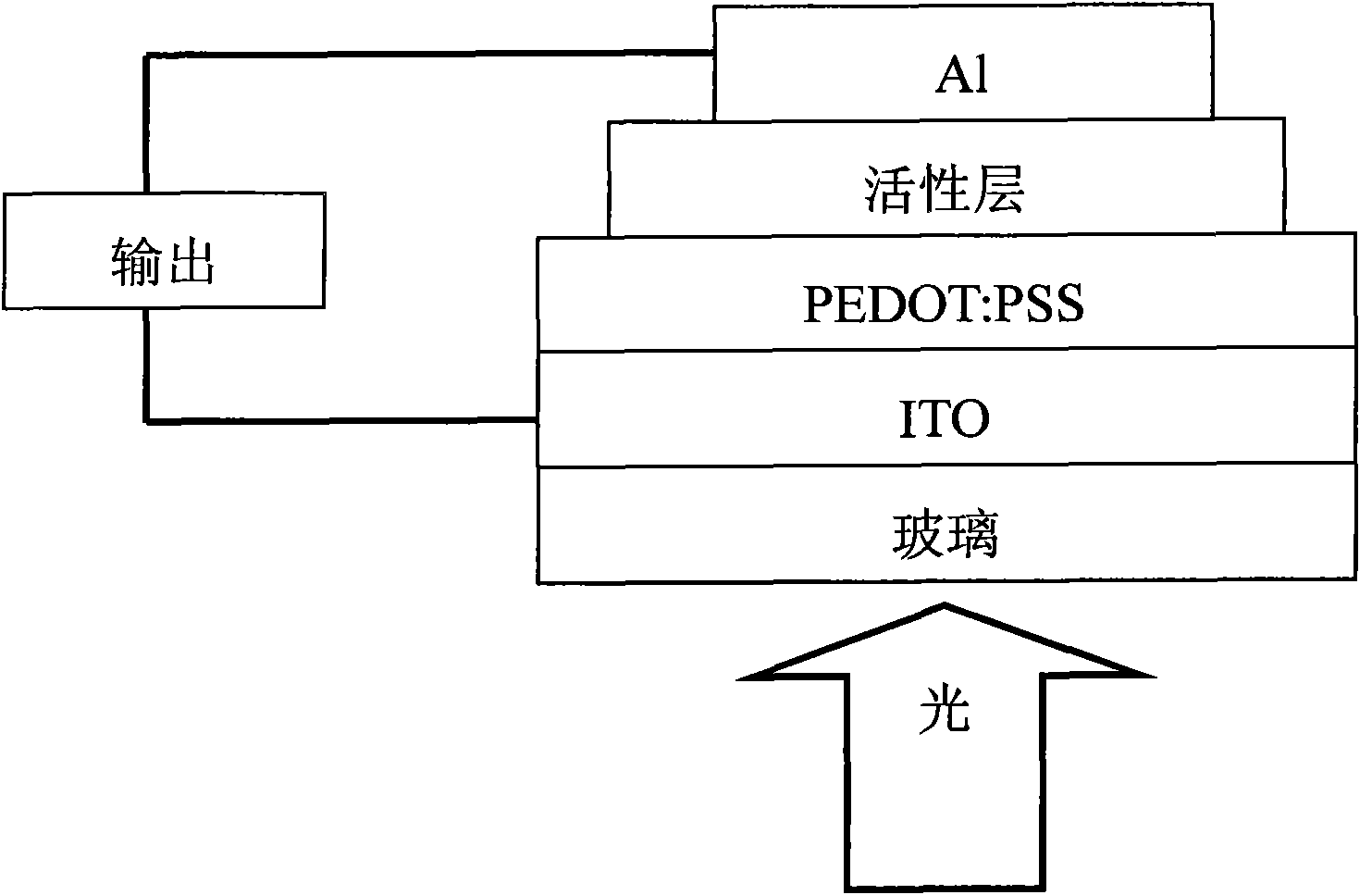
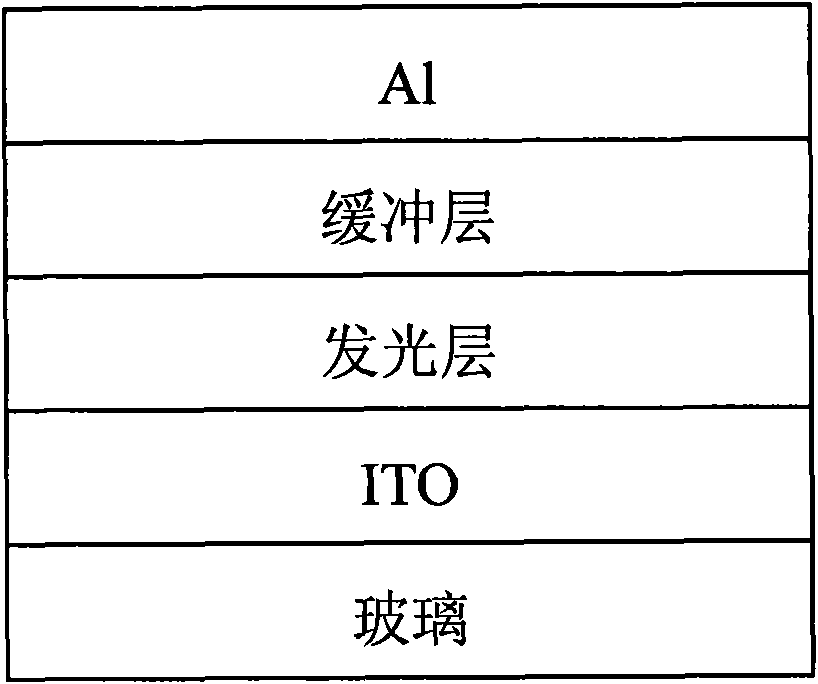
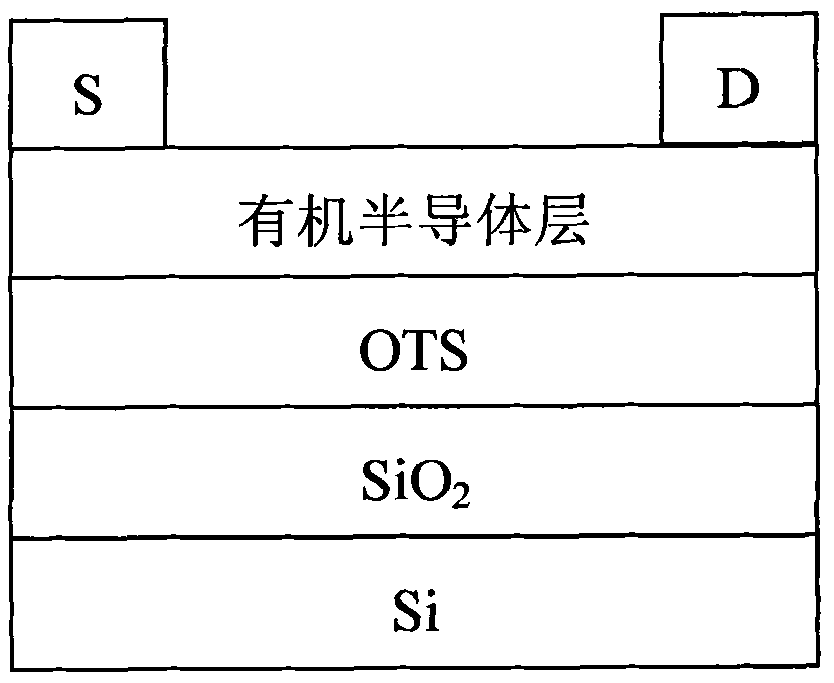
Image
Examples
preparation example Construction
[0035] The present invention also provides a method for preparing a copolymer containing thienylpyrrole diketopic units, comprising the steps of:
[0036] Step S1: Add the thiophene material and n-butyl lithium to the first solvent at a molar ratio of 1:2 to 4 at -100°C to -25°C for reaction, then add trimethyltin chloride and continue the reaction for 24 ~48 hours, obtain the double tin compound of thiophene material;
[0037] Described reaction formula is as follows:
[0038]
[0039] In the formula, m is an integer greater than or equal to 1 and less than or equal to 5; R 1 , R 2 for C 1 -C 20 the alkyl group;
[0040] S2. In an oxygen-free environment, in the presence of a catalyst and a second solvent, mix 1,3-dibromo-5-alkylthiophene[3,4-c]-pyrrole-4,6-dione with the thiophene material The bistannide compound is carried out Stille reaction at a temperature of 65°C to 120°C for 24 to 72 hours at a molar ratio of 1:a to obtain the structural formula: The copolym...
Embodiment 1
[0052] This embodiment discloses a polymer with the following structure:
[0053]
[0054] In the above formula, n=20;
[0055] The preparation steps of above-mentioned polymer are as follows:
[0056] One, the preparation of (3,4-dimethylthiophene-2,5-diyl) bis(trimethyltin):
[0057]
[0058] At -100°C under nitrogen, add 40.00mL (1.00M) n-butyllithium solution into a reaction flask containing 1.12g 3,4-dimethylthiophene and 100mL tetrahydrofuran, stir for 1 hour and then drop slowly Add 7.96g of trimethyltin chloride, return to room temperature, and continue stirring for 24 hours. After the reaction, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, and recrystallized to obtain (3,4-dimethylthiophene-2,5-diyl) bis(trimethyltin) product .
[0059] MALDI-TOF-MS (m / z): 437.8 (M + ).
[0060] Two, the preparation of 5-methylthiophene [3,4-c]-pyrrole-4,6-dione:
[0061]
[0062] At room t...
Embodiment 2
[0073] This embodiment discloses a polymer with the following structure:
[0074]
[0075] In the above formula, n=62;
[0076] The preparation steps of above-mentioned polymer are as follows:
[0077] One, the preparation of (3,4-dioctylthiophene-2,5-diyl) bis(trimethyltin):
[0078]
[0079] At -78°C under argon, add 10.00mL (2.00M) n-butyllithium solution into a reaction flask containing 3.09g 3,4-dioctylthiophene and 110mL tetrahydrofuran, stir for 1 hour and slowly Add 3.98 g of trimethyltin chloride dropwise, return to room temperature, and continue stirring for 36 hours. After the reaction, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, and recrystallized to obtain (3,4-dioctylthiophene-2,5-diyl)bis(trimethyltin) product .
[0080] MALDI-TOF-MS (m / z): 634.2 (M + ).
[0081] Two, the preparation of 5-octylthiophene [3,4-c]-pyrrole-4,6-dione:
[0082]
[0083] Add 5.75 g of thio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com