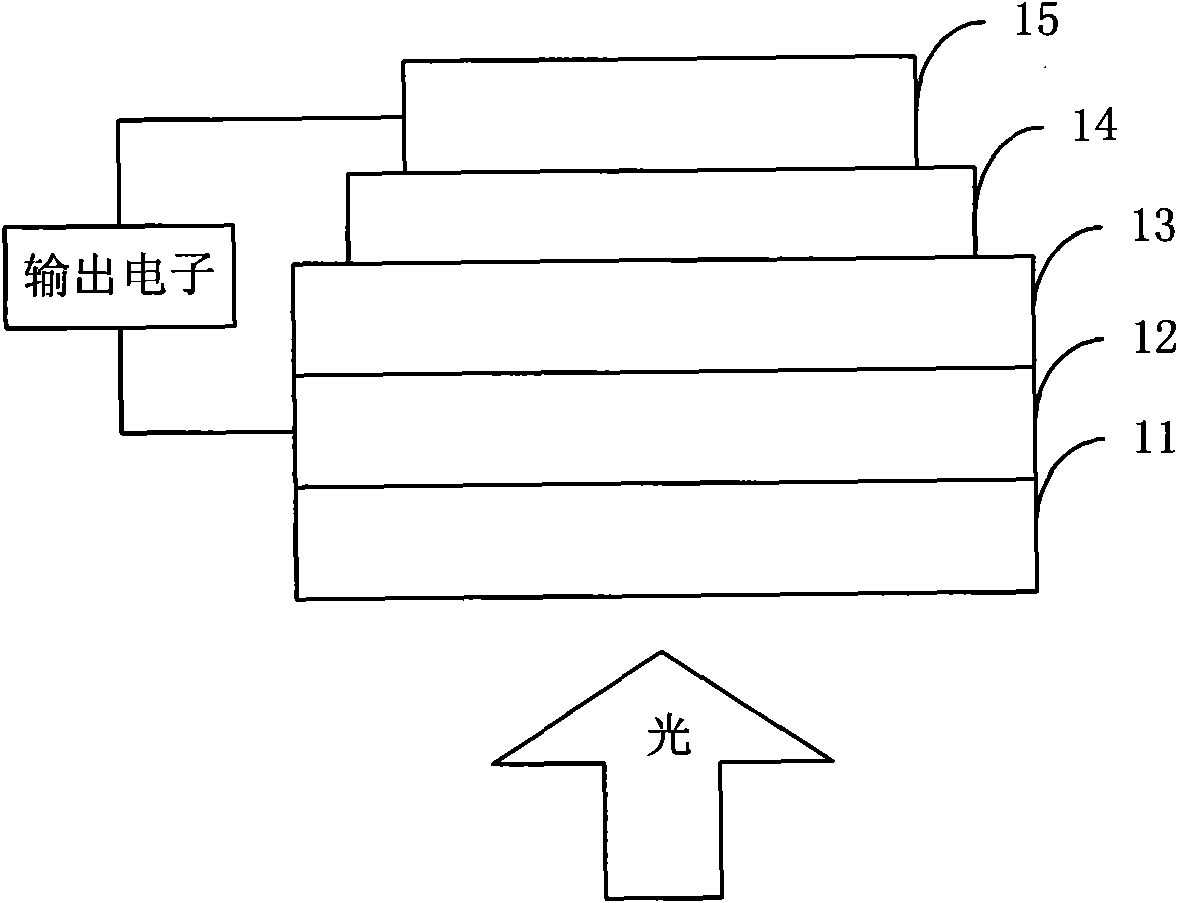
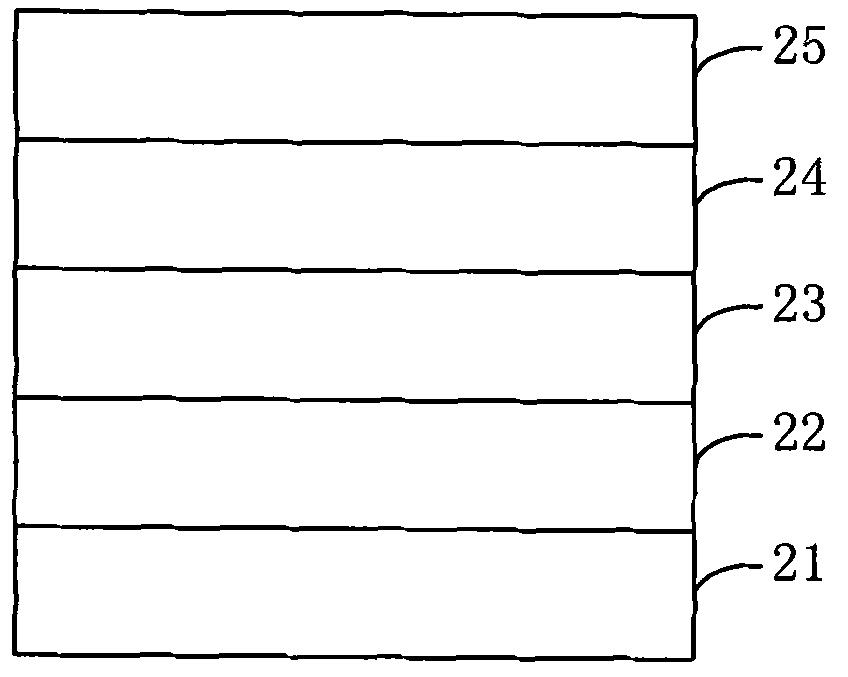
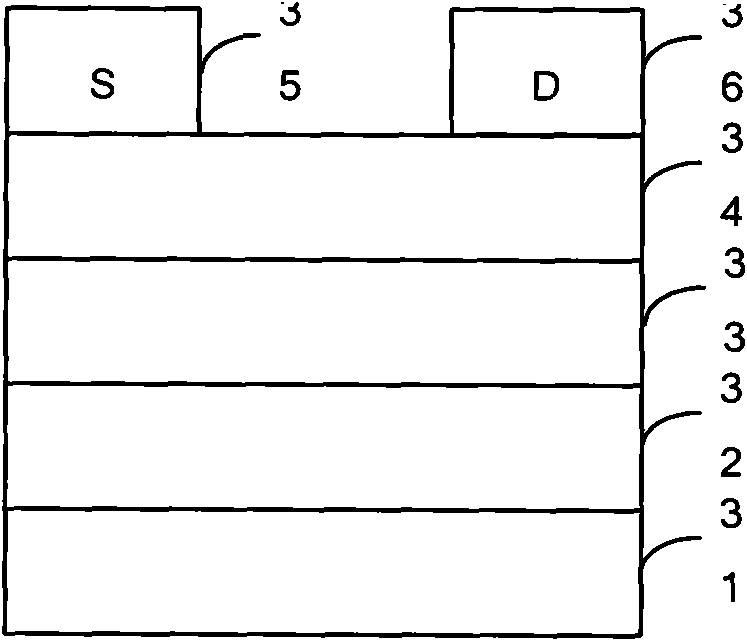
Thiophene organic semiconductor material and preparation method and application thereof
An organic semiconductor and thiophene technology, applied in the field of organic semiconductor materials, can solve the problems of low conversion efficiency of inorganic solar cells, low collection efficiency of carrier electrodes, and ineffective use of the red light region, achieving excellent electrochemical reduction properties, The effect of high electron transport properties and good environmental stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a method for preparing a class of thiophene organic semiconductor materials, comprising the following steps:
[0043] Step S1, the preparation of 5,8-dibromo-2,3-disubstituted-quinoxaline
[0044] Mix and react 3,6-dibromo-o-phenylenediamine and alkyl ethylene dione to obtain 5,8-dibromo-2,3-disubstituted-quinoxaline; the reaction formula is as follows:
[0045]
[0046] Step S2, the preparation of 5,8-bis-(4-alkyl-2-thienyl)-2,3-disubstituted-quinoxaline
[0047] In an oxygen-free environment, the 5,8-dibromo-2,3-disubstituted-quinoxaline, 3-alkyl-2-boronic acid thiophene and anhydrous sodium carbonate were added to the second organic compound containing the second catalyst. In a solvent, the reaction produces 5,8-bis-(4-alkyl-2-thienyl)-2,3-disubstituted-quinoxaline; wherein, the second catalyst is organic palladium or organic palladium and organic palladium A mixture of phosphorus ligands; the second organic solvent is at leas...
Embodiment 1
[0066] Embodiment 1 The structural formula of a class of thiophene organic semiconductor materials in this embodiment is as follows:
[0067]
[0068] In the formula, R 1 , R 4 , R 2 , R 3 for C 1 -C 20 The alkyl group; Its preparation process is as follows:
[0069] 1. 5,8-di-(5-bromo-4-substituted-2-thienyl)-2,3-dialkyl-quinoxaline, the structural formula is as follows:
[0070]
[0071] Now take 5,8-bis-(5-bromo-2-(4-dodecyl)thienyl)-2,3-dioctyl-quinoxaline as an example for illustration.
[0072] 1), 5,8-dibromo 2,3-dioctyl-quinoxaline, the structural formula is as follows:
[0073]
[0074] Take the preparation of 5,8-dibromo-2,3-dioctylquinoxaline as an example to illustrate, and the preparation process is as follows:
[0075]
[0076] 3,6-Dibromo-o-phenylenediamine (0.5 g, 1.85 mmol) was added to a solution of the compound dioctylethylenedione (0.28 g, 1 mmol) in acetic acid (30 mL) at 120°C. Reflux overnight, pour the reaction solution into water, ...
Embodiment 2
[0114] Embodiment 2 The structural formula of a class of thiophene organic semiconductor materials in this embodiment is as follows:
[0115]
[0116] In the formula, R 1 , R 4 is an alkyl group, R 2 , R 3 for C 1 -C 20 Alkyl substituted phenyl; Its preparation process is as follows:
[0117] 1. 5,8-bis-(5-bromo-2-thienyl)-2,3-diphenyl-quinoxaline
[0118]
[0119] The preparation of 5,8-bis-(5-bromo-4-n-eicosyl-2-thienyl)-2,3-diphenyl-quinoxaline will now be described as an example. The preparation process is as follows:
[0120]
[0121] Under nitrogen atmosphere, NBS (0.6g, 3.3mmol) was added to 5,8-di(4-n-dodecyl-2-thienyl)-2,3-dioctyl-quinoxaline (1.2 g, 1.53mmol) in tetrahydrofuran (THF 50mL) and stirred overnight at room temperature. The reaction solution was spin-dried and the crude product was obtained by column chromatography with a yield of 73%. MS (MALDI) m / z: 941 (M + )
[0122] Among them, the preparation of 4,4-dialkyl-2,6-bistrimethyltin-cy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com