Preparation method of medical metal implanted material porous niobium
An implant material, porous niobium technology, applied in the field of porous medical metal implant materials, can solve the problems of insufficient purity of finished products, carbon skeleton residues, and reduced biological safety, and achieve the goals of reducing impurity content, uniform quality, and increasing porosity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
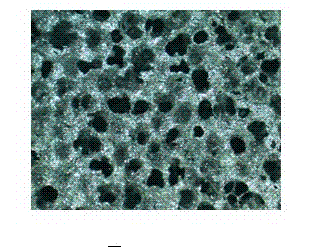
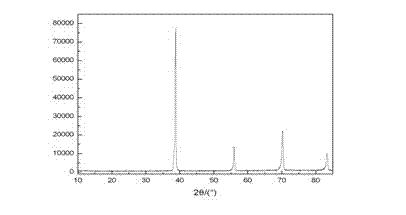
Image
Examples
Embodiment 1
[0033] Example 1: Weigh 3 g of ethyl cellulose and put it into a container containing 240 ml of absolute ethanol; place it on an electric stove to heat and stir to make it an ethyl cellulose ethanol solution. Use a 200g balance to weigh 60g of niobium powder with an average particle size of less than 38 microns and an oxygen content of less than 0.1%, add 15ml of cooled ethyl cellulose ethanol solution, stir and mix evenly, and make it into a niobium powder slurry. Use 10×10×30mm porous polyurethane foam (average pore diameter is 0.48mm, density 0.025g / cm 3 , hardness 50°) into it and pour until the pores of the polyurethane foam are filled with niobium powder slurry, then use a clip to take out the polyurethane foam filled with niobium powder slurry and put it into a porcelain plate. Dry in a vacuum drying oven at a drying temperature of 60° C., a drying time of 6 hours, and a vacuum degree of 1 Pa. Degreasing treatment: the vacuum degree is lower than 1×10 -3 Pa, temperatu...
Embodiment 2
[0036] Example 2: Weigh 5 g of ethyl cellulose and put it into a container containing 200 ml of absolute ethanol; place it on an electric stove to heat and stir to make it an ethyl cellulose ethanol solution. Use a 200g balance to weigh 40g of niobium powder with an average particle size of less than 38μm and an oxygen content of less than 0.1%, add 10ml of ethyl cellulose ethanol solution, stir and mix evenly, and make it into a niobium powder slurry. Use 10×10×25mm porous polyurethane foam (average pore diameter is 0.56mm, density 0.030g / cm 3 , hardness 60 0) into it and pour until the pores of the polyurethane foam are filled with the niobium powder slurry, and the polyurethane foam filled with the niobium powder slurry is taken out with a clip and put into the porcelain plate. Dry in a vacuum drying oven, the drying temperature is 70 ° C, the drying time is 5 hours, and the vacuum degree is kept at 1×10 -2 Pa. Degreasing treatment: the vacuum degree is lower than 1×10 ...
Embodiment 3
[0039] Example 3: Weigh 6 g of ethyl cellulose and put it into a container containing 220 ml of absolute ethanol; place it on an electric stove to heat and stir to make it an ethyl cellulose ethanol solution. Use a 200g balance to weigh 45g of niobium powder with an average particle size of less than 38μm and an oxygen content of less than 0.1%, add 12ml of ethyl cellulose ethanol solution, stir and mix evenly, and make it into a niobium powder slurry. Use 8×8×25mm porous polyurethane foam (average pore size is 0.70mm, density 0.035g / cm 3 , hardness 70°) into it and pour until the pores of the polyurethane foam are filled with niobium powder slurry, then use a clip to take out the polyurethane foam filled with niobium powder slurry and put it into a porcelain plate. Dry in a vacuum drying oven, the drying temperature is 50 ° C, the drying time is 6 hours, and the vacuum degree is kept at 1×10 -1 Pa. Degreasing treatment: the vacuum degree is lower than 1×10 -3 Pa, temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Average pore diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com