Diamine monomer, synthetic method thereof, and polyimide prepared from diamine monomer
A diamine monomer and synthesis method technology, applied in the field of fluorine-containing polyimide, can solve the problems of limited application, high melting point or softening temperature, poor solubility, etc., and achieve reduced water absorption, good moisture-sensitive performance, and reduced crystallization The effect of strength and rigidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
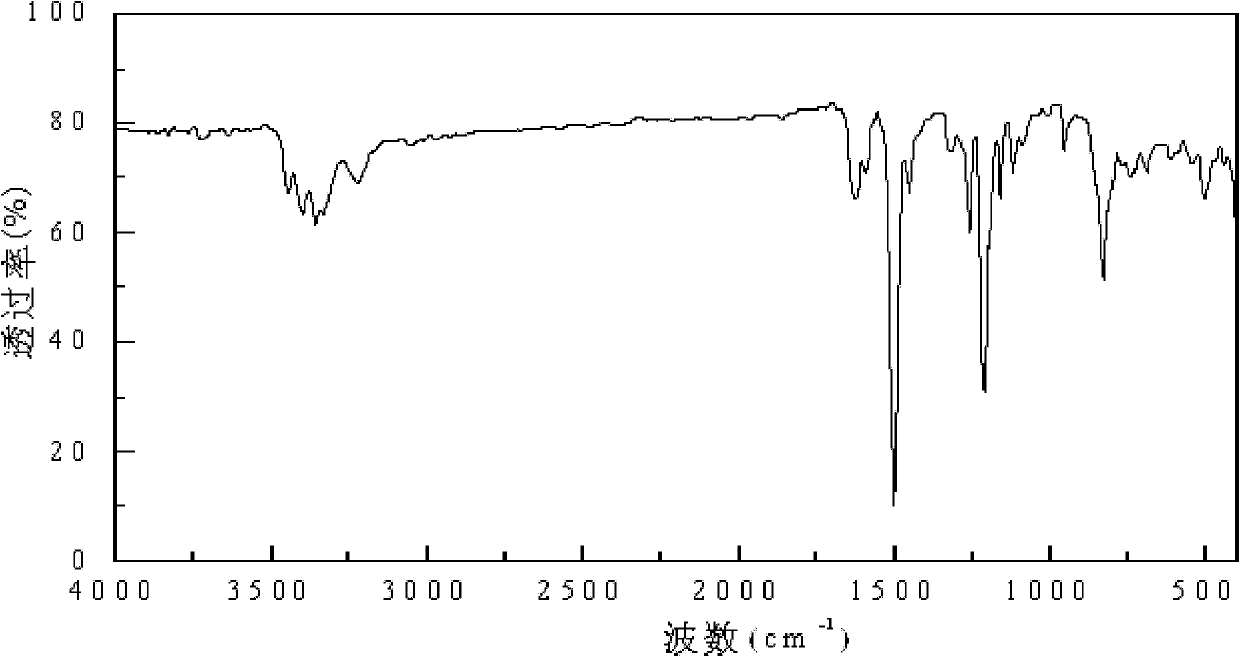
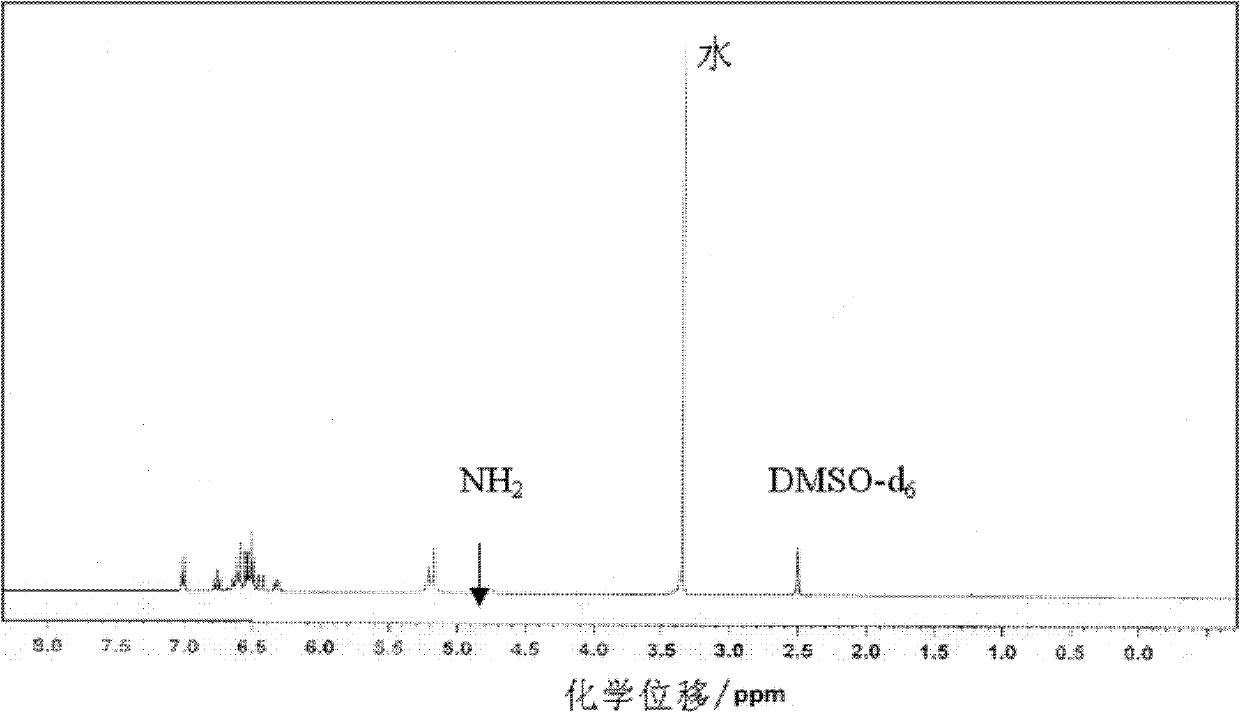
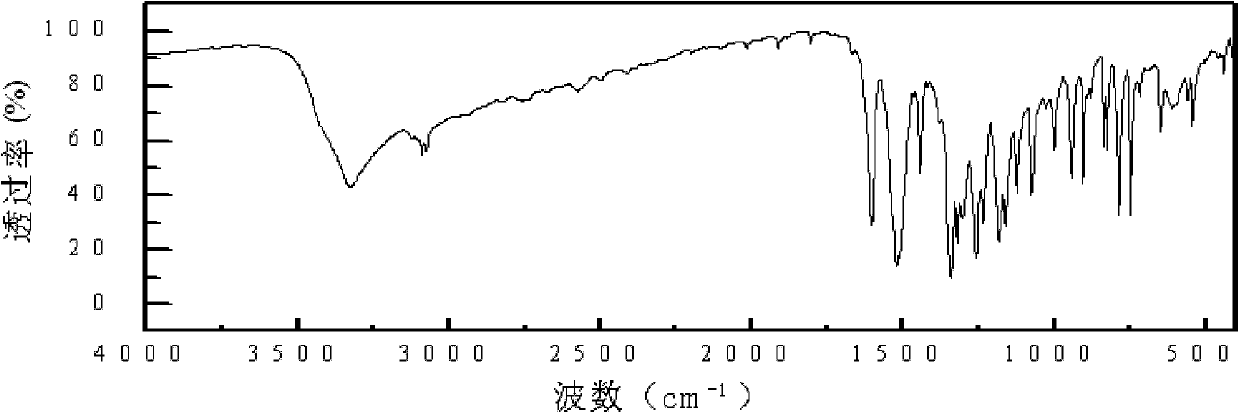
[0026] Specific embodiment 1: This embodiment is a diamine monomer, the chemical name of the diamine monomer is 4-(4-aminophenoxy)-3-fluoroaniline, and the structural formula is as follows:
[0027]
[0028] The 4-(4-aminophenoxy)-3-fluoroaniline diamine monomer of this embodiment is a diamine monomer with a fluorine-containing asymmetric aromatic ether structure. It is a white to light yellow solid and is a good synthetic polyamide. The raw material of imine.
specific Embodiment approach 2
[0029] Embodiment 2: This embodiment is the method for synthesizing the diamine monomer described in Embodiment 1, which is obtained through the following steps: 1. Synthesis of 2-fluoro-4-nitro-1-(4-nitro (Phenoxy) benzene: Add 2-fluoro-4-nitrophenol, 4-nitrochlorobenzene, anhydrous potassium carbonate and N,N-dihydrogen to a reactor equipped with a magnet, a thermometer and a condenser. Methylformamide (DMF), then place the reactor in an oil bath at 120°C to 160°C, keep it warm and stir and reflux for 10 to 15 hours to obtain the reaction system, then filter the reaction system while it is hot, and then add the filtrate Add distilled water at 5℃~15℃ until no solid matter is precipitated, and then recrystallize with absolute ethanol to obtain 2-fluoro-4-nitro-1-(4-nitrophenoxy)benzene (light yellow needles) Crystal), wherein the molar ratio of 2-fluoro-4-nitrophenol and 4-nitrochlorobenzene is 1:1, and the molar ratio of anhydrous potassium carbonate and 4-nitrochlorobenzene i...
specific Embodiment approach 3
[0042] Specific embodiment three: This embodiment is different from the specific embodiment two in that the molar ratio of anhydrous potassium carbonate and 4-nitrochlorobenzene in step one is 1.1:1. Other steps and parameters are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wave number | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com