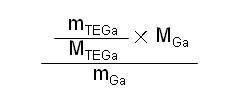Method for one-step process preparation of high-purity triethyl gallium
A triethylgallium, high-purity technology is applied in the field of one-step preparation of high-purity triethylgallium, which can solve the problems of low reaction conversion rate, high raw material cost, affecting material purity, etc., and achieves cheap raw materials and reaction yield. High and convenient purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
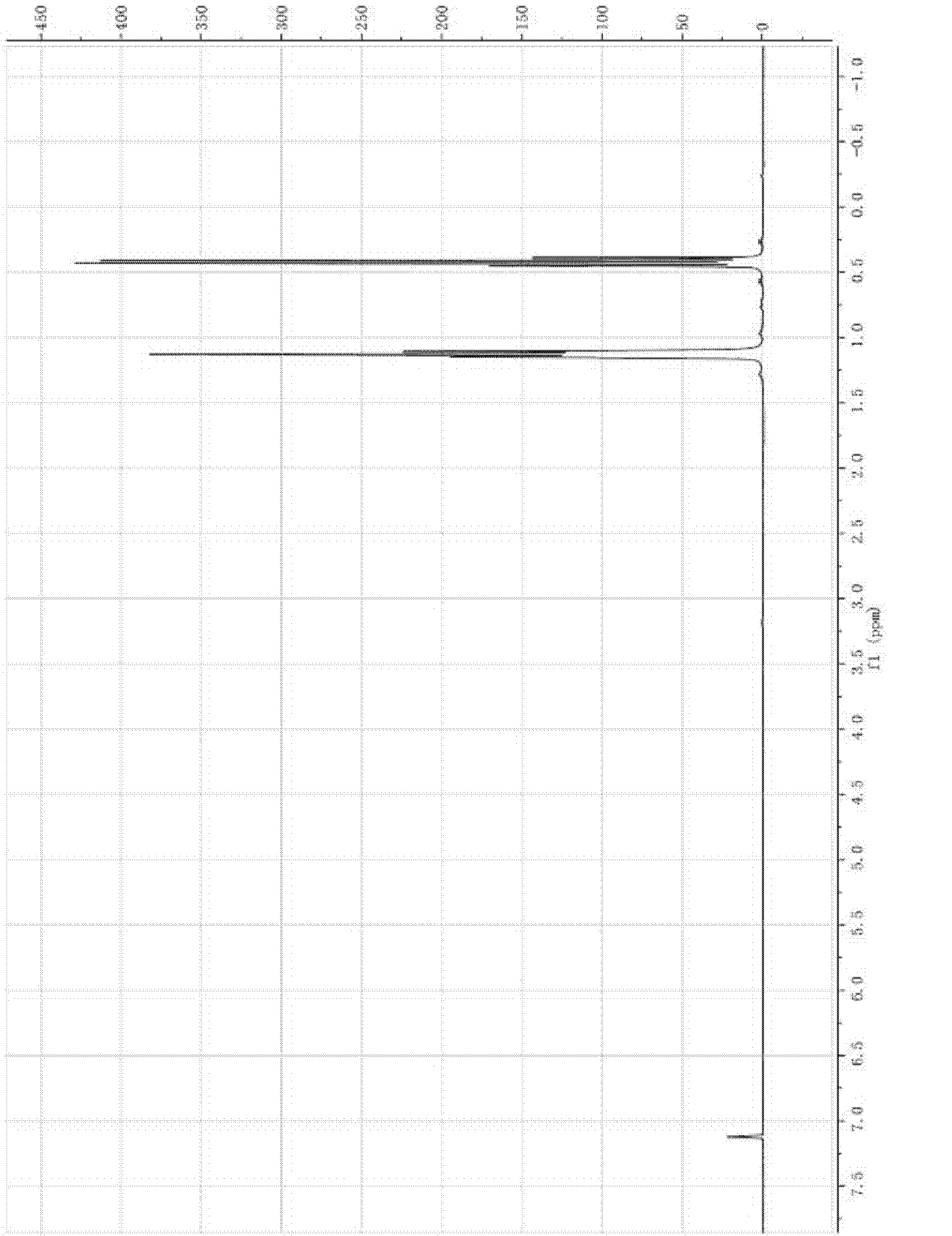
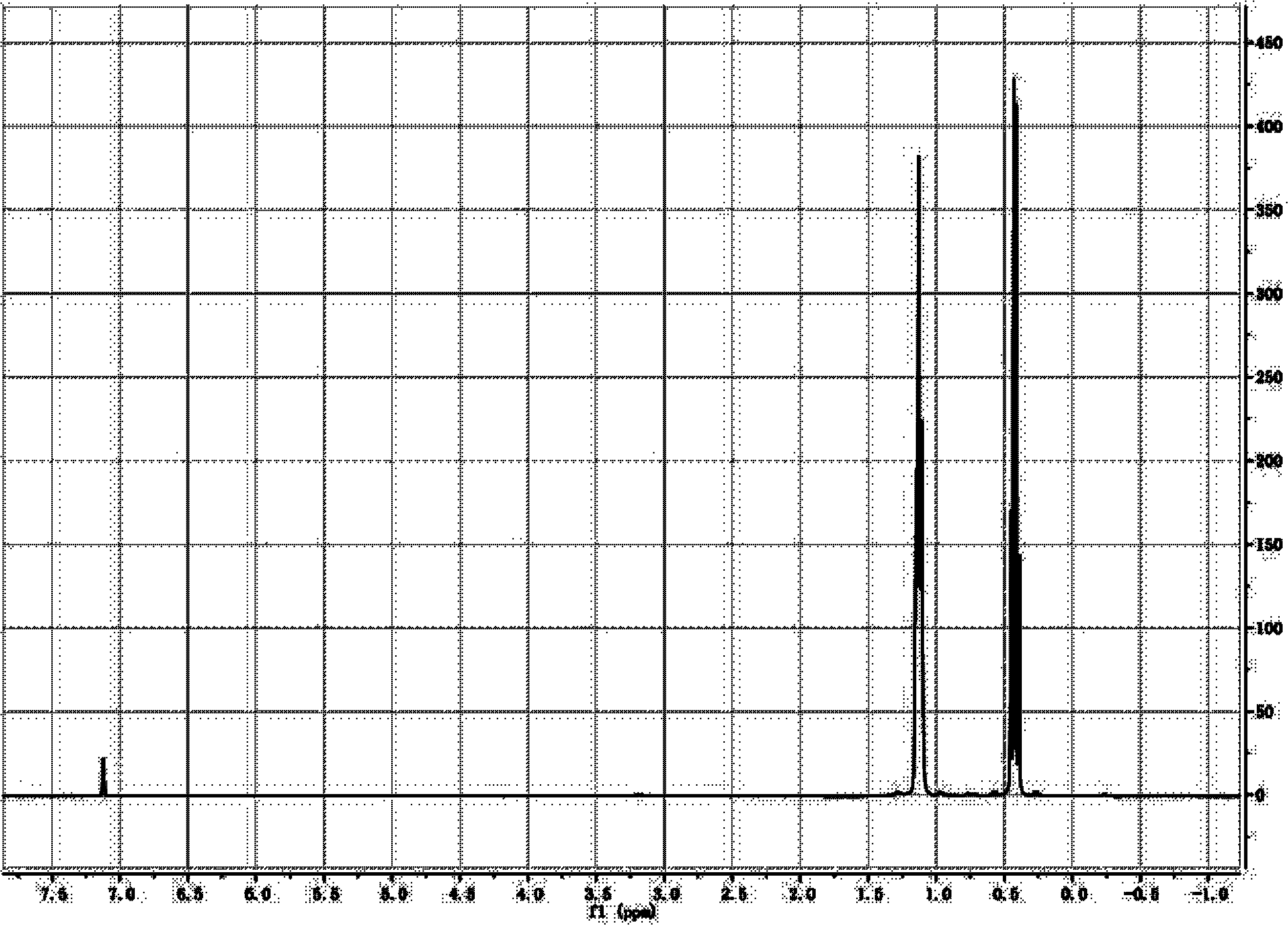
[0021] 600g gallium-magnesium alloy Ga x Mg y and metallic magnesium Mg z Put it into the reaction kettle and protect it with inert gas. Wherein x=0.2, y=0.4, z=0.4, x+y+z=1, wherein x, y, z are molar ratios. Add 1,300g of tetraethylene glycol dimethyl ether, and gradually add 2,000g of ethyl iodide dropwise under normal temperature stirring conditions. After the reaction is completed, continue to reflux at a temperature of 100-150°C for 6 hours, distill out low boiling point substances, and then continue to increase the temperature The decomposition temperature was controlled at 160-220°C to obtain 369g of high-purity triethylgallium with a yield of 65% (calculated based on the mass of gallium metal).
[0022] The yield is calculated as follows:
[0023]
[0024] in:
[0025] m TEGa In order to obtain the quality of high-purity triethylgallium,
[0026] m TEGa is the molecular weight of triethylgallium,
[0027] m Ga is the molecular weight of gallium,
[0028] ...
Embodiment 2
[0030] 600g gallium-magnesium alloy Ga x Mg y and metallic magnesium Mg z Put it into the reaction kettle and protect it with inert gas. Wherein x=0.25, y=0.5, z=0.25, x+y+z=1, wherein x, y, z are molar ratios. Add 1,300g of tetraethylene glycol dimethyl ether, and gradually add 2,000g of ethyl iodide dropwise under normal temperature stirring conditions. After the reaction is completed, continue to reflux at a temperature of 100-150°C for 6 hours, distill out low boiling point substances, and then continue to increase the temperature The decomposition temperature was controlled at 160-220°C to obtain 511g of high-purity triethylgallium with a yield of 77% (calculated based on the mass of gallium metal).
Embodiment 3
[0032] 600g gallium-magnesium alloy Ga x Mg y and metallic magnesium Mg z Put it into the reaction kettle and protect it with inert gas. Wherein x=0.3, y=0.4, z=0.3, x+y+z=1, wherein x, y, z are molar ratios. Add 1,300g of tetraethylene glycol dimethyl ether, and gradually add 2,000g of ethyl iodide dropwise under normal temperature stirring conditions. After the reaction is completed, continue to reflux at a temperature of 100-150°C for 6 hours, distill out low boiling point substances, and then continue to increase the temperature The decomposition temperature was controlled at 160-220°C to obtain 508g of high-purity triethylgallium with a yield of 68% (calculated based on the mass of gallium metal).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com