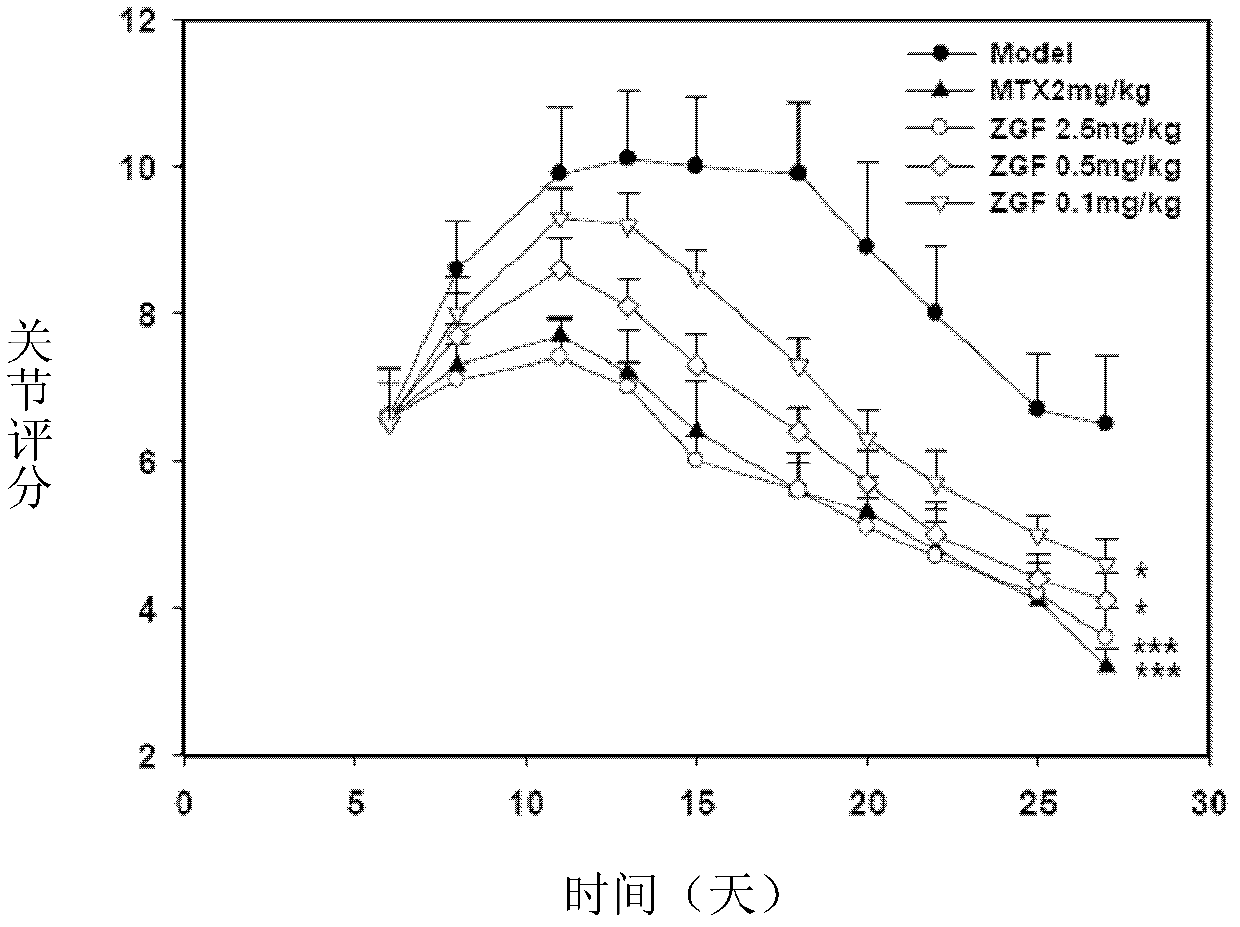
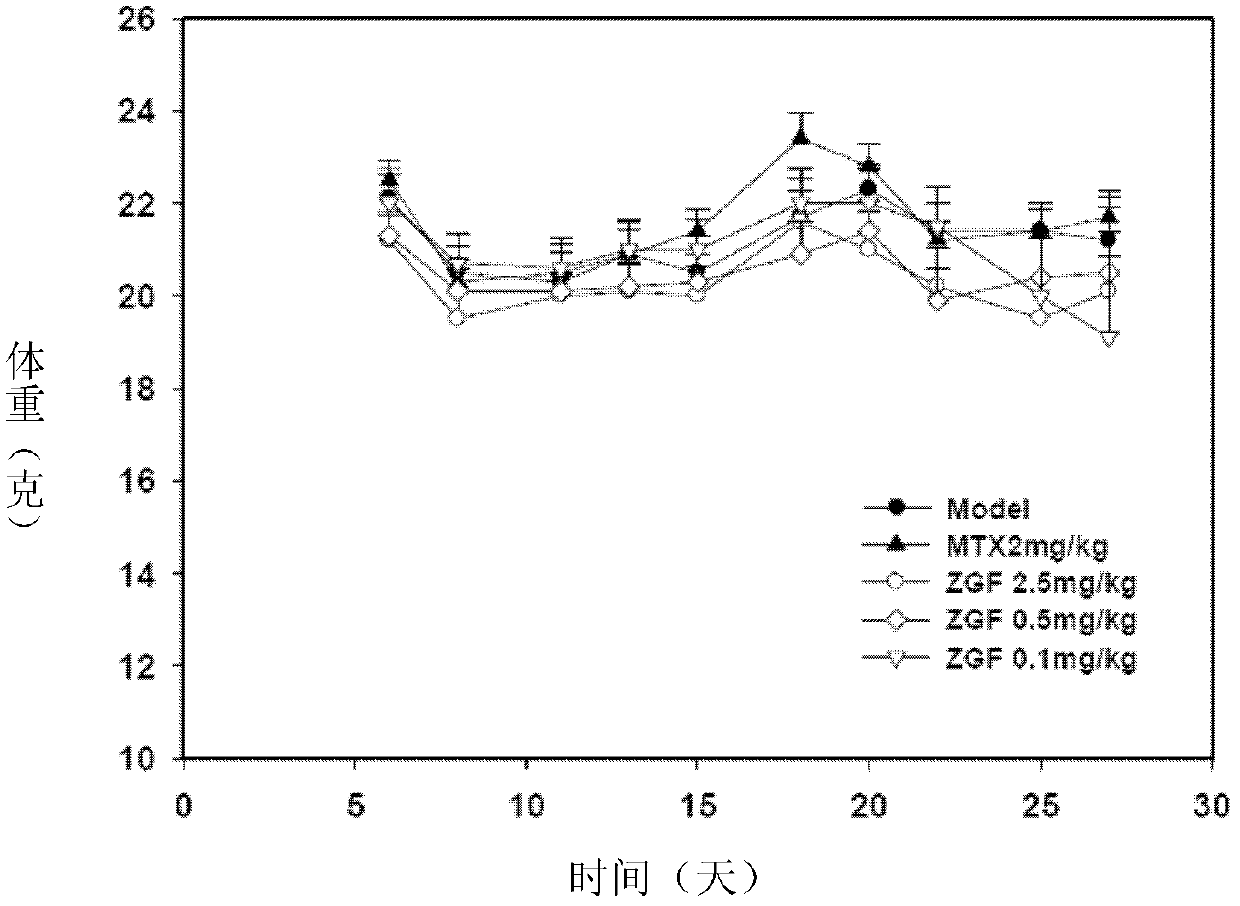
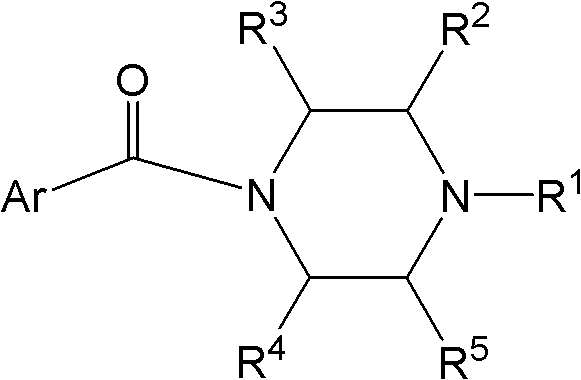
Novel application of piperazine acidamide compound in pharmacy
A technology of piperazine amides and compounds, which is applied in the new application field of piperazine amides in pharmaceuticals, and can solve the problems of unapplied rheumatoid arthritis prevention and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation of 3-cyano-4-methyl-6-phenylpyridazine (compound 2)
[0057]
[0058]Add 1.25g (6.1mol) 3-chloro-4-methyl-6-phenylpyridazine, 0.99g (11.0mol) cuprous cyanide and 20mL pyridine into a 50mL pressure-resistant reaction tube, and react at 130°C for 24 hours , cooled, filtered, the filter cake was washed 3 times with 50 mL of ethyl acetate, and the organic phase was washed twice with 100 mL of 1N HCl, then dried with anhydrous sodium sulfate, and concentrated to obtain the crude product of compound 2 as a brown solid, which can be directly obtained without purification. For the next reaction, MS: 196.0 (M+H + ).
Embodiment 23
[0059] The preparation of embodiment 23-carboxy-4-methyl-6-phenylpyridazine (compound 3)
[0060]
[0061] Compound 2 prepared in Example 1 was added to 20 mL of 8N HCl solution, heated to reflux for 4 hours, neutralized with saturated sodium bicarbonate after cooling to make it a sodium salt, extracted twice with ethyl acetate, and the aqueous phase was Adjust the pH to 2 with dilute hydrochloric acid, then extract 3 times with ethyl acetate, dry over anhydrous sodium sulfate, and concentrate to obtain 0.59 g of light yellow solid compound 3, yield 55%, MS: 213.0 (M-H + ).
Embodiment 3
[0062] Example 3 Preparation of (4-(4-fluorophenyl)piperazinyl)(4-methyl-6-phenylpyridazine)methanone (compound 4)
[0063]
[0064] In a 50ml round bottom flask, add 0.40g (1.87mol) of compound 3 and 20ml of dry dichloromethane, then add 0.30g (2.24mol) of HOBt, 0.43g (2.24mol) of EDC hydrochloride, 0.23g of triethyl Amine and 0.34 g (1.87 mol) of 1-(4-fluorophenyl)piperazine. The mixture was stirred at room temperature for 18 h, and then the dichloromethane was removed by rotary evaporation. The obtained oil was extracted with 100 ml of ethyl acetate and 50 ml of saturated sodium bicarbonate solution, and the organic layer was dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography. Obtained 40.49 g of white solid compound, 69%.
[0065] 1 H-NMR (400MH, CDCl 3 )δ8.10-8.12 (m, 2H), 7.77 (s, 2H), 7.53-7.57 (m, 3H), 6.97-7.01 (m, 2H), 6.89-6.93 (m, 2H), 4.06 (t, J=4.8Hz, 2H), 3.59(t, J=4.8Hz, 2H), 3.14(t, J=4.8Hz, 2H), 2.49(s, 3H); MS: 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com