Method for preparing ethylene carbonate through continuous heterogeneous catalysis and catalyst
A heterogeneous catalyst, ethylene carbonate technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of high catalyst cost, catalyst deactivation, separation and other problems, to achieve the effect of high catalyst activity, simple operation and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Synthesis of catalyst-imidazolium ionic liquid polymer immobilized on inorganic powder:
[0046] (a) Take a three-necked flask and feed it with N 2 For protection, add 9.41g N-vinylimidazole (0.1mol) and 30g absolute ethanol and heat and stir, then slowly add 12.52g bromoethanol (0.1mol) dropwise, heat and stir at 70°C for 24h, distill under reduced pressure, and dry in vacuo to obtain Vinylimidazolium Ionic Liquid.
[0047] (b) After mixing 10 parts of vinylimidazole ionic liquid, 5 parts of methacrylic acid, 1 part of styrene, 10 parts of hydroxyethyl acrylate and 65 parts of double distilled water, based on the weight of the above mixture, the The imidazolium ionic liquid polymer was prepared by copolymerization under the action of 2% initiator ammonium persulfate, 1% sodium hypophosphite monohydrate, 1‰ tetrabutyl ammonium bromide, and 2‰ sodium dodecyl sulfate for 2 hours.
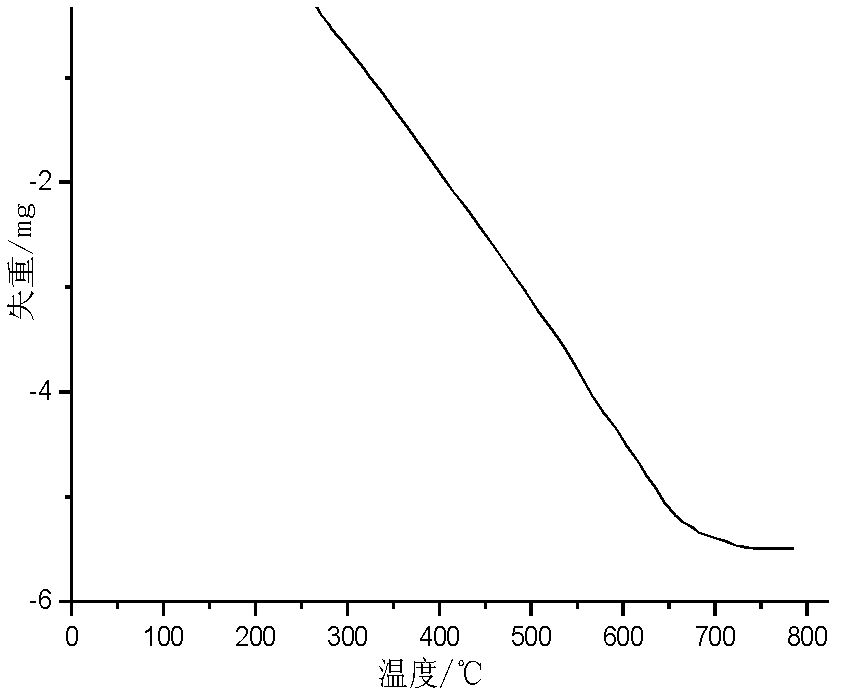
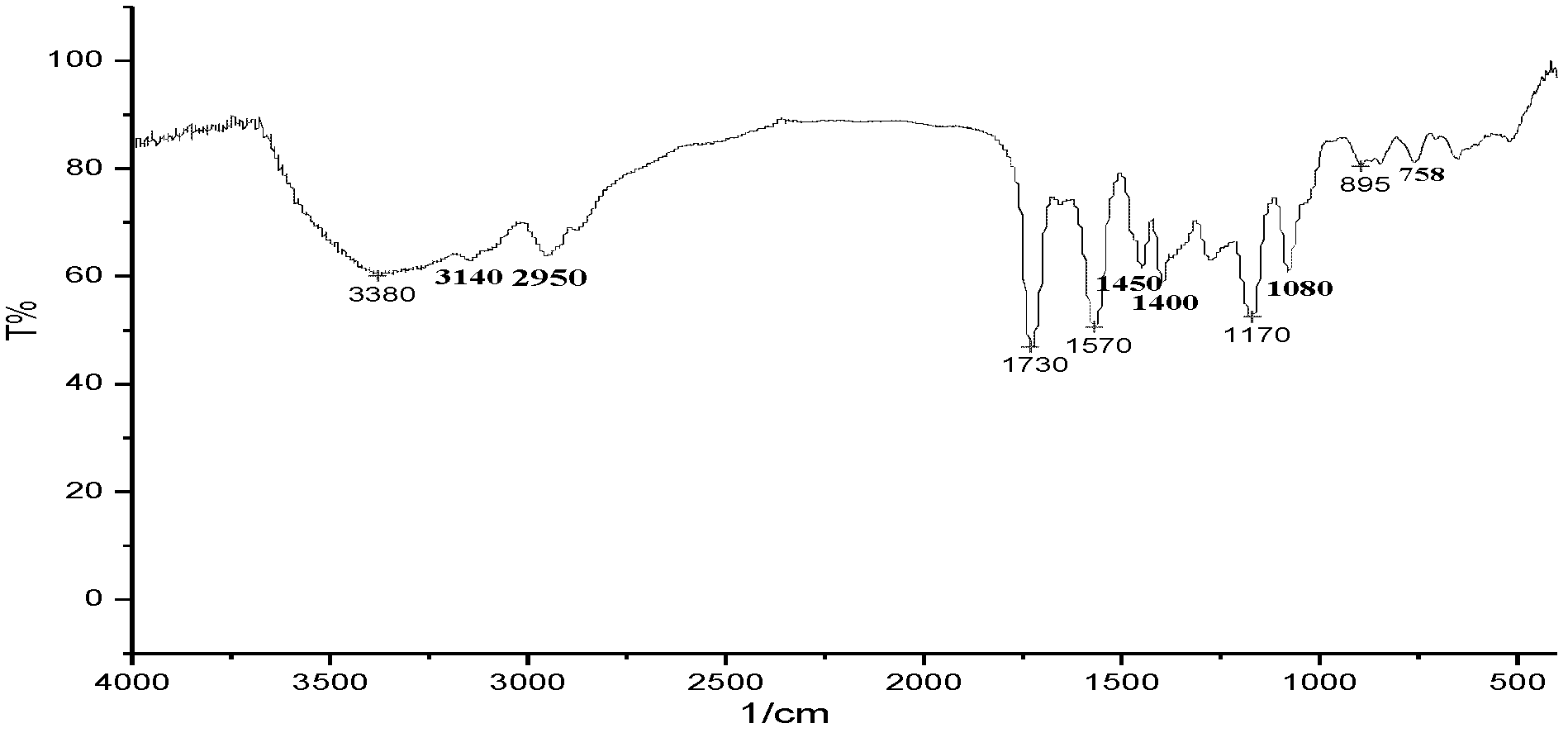
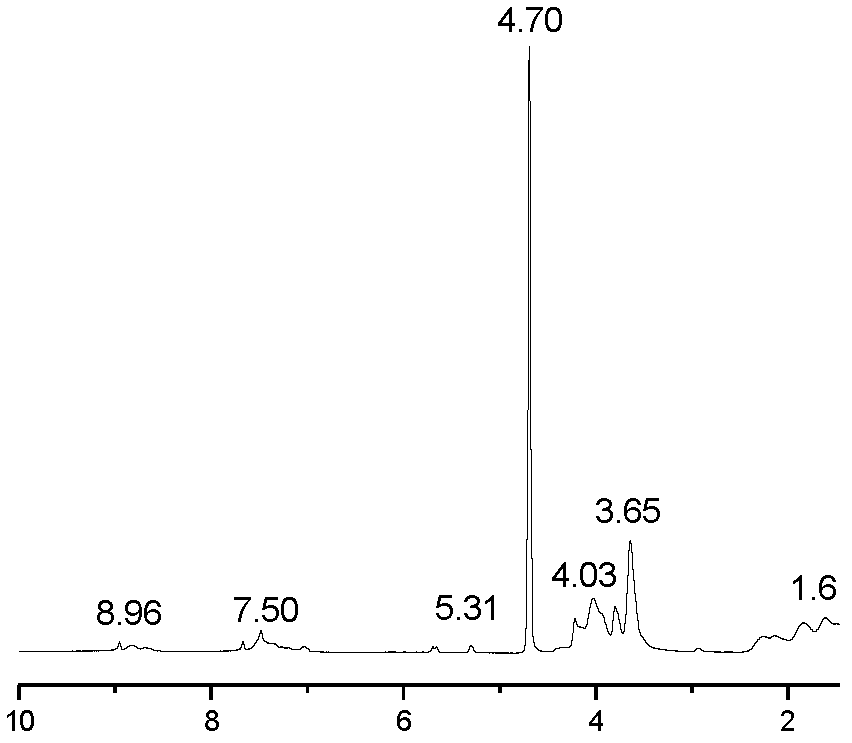
[0048] Thermogravimetry curve, infrared spectrum and 1 HNMR figure (nuclear magnetic...
Embodiment 2
[0052] (1) Synthesis of catalyst-imidazolium ionic liquid polymer immobilized on inorganic powder:
[0053] (a) Take a three-necked flask and feed it with N 2 For protection, add 9.41g N-vinylimidazole (0.1mol) and 30ml absolute ethanol and heat and stir, then slowly add 12.42g bromoethylamine (0.1mol) dropwise, heat and stir at 70°C for 24h, then distill under reduced pressure, vacuum Dry to obtain vinylimidazolium ionic liquid.
[0054] (b) After mixing 10 parts of vinylimidazole ionic liquid, 5 parts of methacrylic acid, 1 part of styrene, 10 parts of hydroxyethyl acrylate and 65 parts of double distilled water, based on the weight of the above mixture, the The imidazolium ionic liquid polymer was prepared by copolymerization under the action of 2% initiator ammonium persulfate, 1% sodium hypophosphite monohydrate, 1‰ tetrabutyl ammonium bromide, and 2‰ sodium dodecyl sulfate for 2 hours.
[0055] (c) The prepared imidazolium ionic liquid polymer was coated on 4A molecula...
Embodiment 3
[0058] (1) Catalyst - synthesis of imidazolium ionic liquid polymer immobilized on inorganic powder:
[0059] (a) Take a three-necked flask and feed it with N 2 For protection, add 9.41g N-vinylimidazole (0.1mol) and 30 parts of absolute ethanol and heat and stir, then slowly add 12.42g bromoethane (0.1mol) dropwise, heat and stir at 70°C for 24h, then distill under reduced pressure, vacuum Dry to obtain vinylimidazolium ionic liquid.
[0060] (b) After mixing 10 parts of vinylimidazole ionic liquid, 5 parts of methacrylic acid, 1 part of styrene, 10 parts of hydroxyethyl acrylate and 65 parts of double distilled water, based on the weight of the above mixture, the The imidazolium ionic liquid polymer was prepared by copolymerization under the action of 2% initiator ammonium persulfate, 1% sodium hypophosphite monohydrate, 1‰ tetrabutyl ammonium bromide, and 2‰ sodium dodecyl sulfate for 2 hours.
[0061] (c) The prepared imidazolium ionic liquid polymer is coated on 4A mole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com