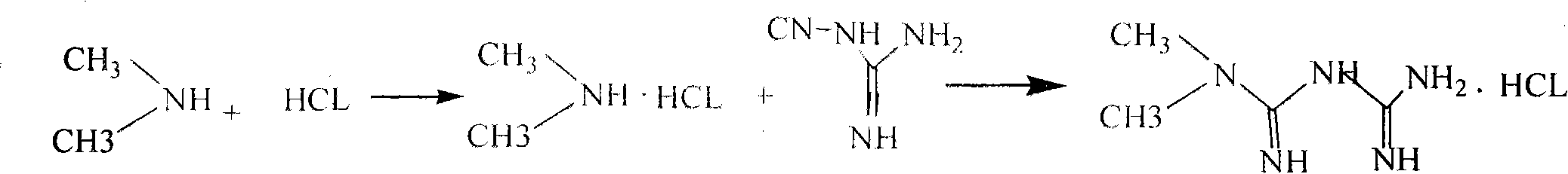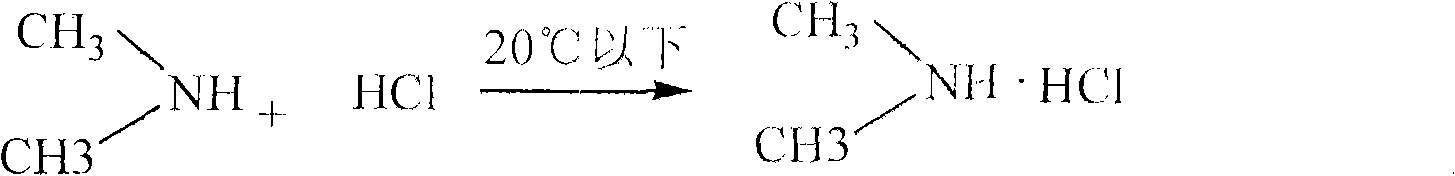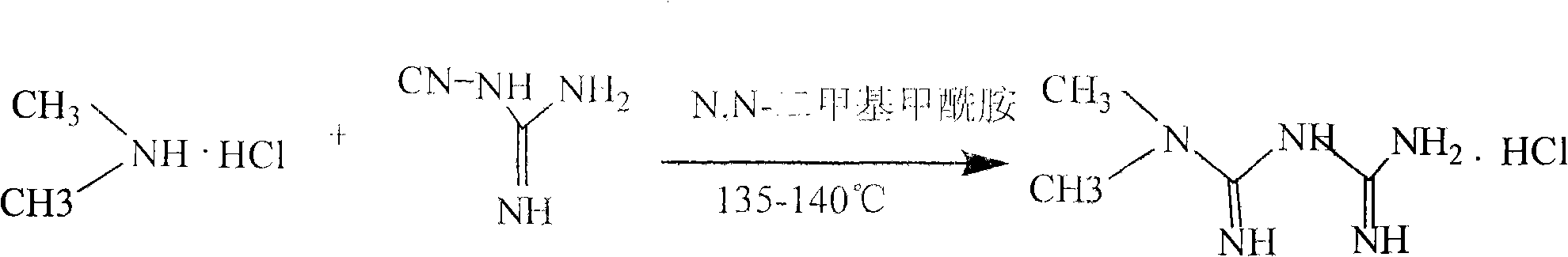Preparation method of metformin hydrochloride
A technology of metformin hydrochloride and dimethylamine hydrochloride, which is applied in the field of easy-to-operate metformin hydrochloride production, high purity, and low cost, can solve the problems of high production cost, high market price, and difficulty in removal, and achieve simple and easy production operation, low solvent Cheap and easy to obtain, the effect of high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A preparation method of metformin hydrochloride, comprising the steps of:
[0025] (1) Salt-forming reaction:
[0026] With 40% dimethylamine as the starting material, dimethylamine hydrochloride is generated through a salt-forming reaction with hydrochloric acid;
[0027] The reaction formula is as follows:
[0028]
[0029] (2) Addition reaction:
[0030] Addition reaction with dicyandiamide in solvent N,N-dimethylformamide generates metformin hydrochloride crude product, and obtains metformin hydrochloride through 80% ethanol refining;
[0031] The reaction formula is as follows:
[0032]
[0033] (3) Refined:
[0034] Metformin hydrochloride is obtained through refining with 80% ethanol; the total yield is 75%-78% based on dimethylamine;
[0035] in:
[0036] Step (1) Salt-forming reaction Add 40% dimethylamine solution into the reaction kettle, lower the temperature to below 20°C, then add concentrated hydrochloric acid solution dropwise for salt-formin...
Embodiment 1
[0040] Salt formation reaction: Use a vacuum pump to pump 520Kg of dimethylamine aqueous solution into the reaction tank, start stirring, pass cold brine through the jacket, cool down to below 20°C, and control the temperature below 20°C, start adding 500Kg of hydrochloric acid dropwise, then measure the pH value, If pH=1-2, stop adding hydrochloric acid dropwise, if pH≥2, continue to add hydrochloric acid dropwise until pH=1-2. After the dropwise addition, keep the reaction at 20-25°C for 12h. After the heat preservation is completed, heat up and distill under reduced pressure. After 590Kg of water is distilled, the mother liquor of the previous batch is pumped into the reaction tank with a vacuum pump, and the temperature is lowered to below 40°C by cooling water, and the temperature is lowered to 10°C by passing cold brine. After the crystallization is completely precipitated, put the material into the centrifuge, filter it, and remove the liquid by centrifugal filter until...
Embodiment 2
[0044] Salt-forming reaction and refining are the same as in Example 1.
[0045] Addition reaction: Use a vacuum pump to pump 520.0Kg of N,N-dimethylformamide into the reaction tank, start stirring, and evenly add 260Kg of dimethylamine hydrochloride and 375Kg of dicyandiamide from the feeding port. After the feeding is completed, the tank is sealed, and the interlayer starts to heat up with steam; the temperature is raised to 135-140°C, and the temperature is kept for 12 hours; After the crystallization is completely precipitated, put the material into the centrifuge, filter it, and remove the liquid by centrifugal filter until no liquid flows out, spread the wet material evenly on the baking tray with a thickness of 2-4cm, and dry it at 90-100°C for 10- After 12 hours, 456.0 kg of metformin hydrochloride was obtained, with a yield of 86.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com