Nitrogen oxide oxidizing catalyst and preparation method of nitrogen oxide oxidizing catalyst
A technology of nitrogen oxides and catalysts, which is applied in the field of catalysts for oxidation and removal of nitrogen oxides. It can solve the problems of poor activity, high cost, and high energy consumption in the field of preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
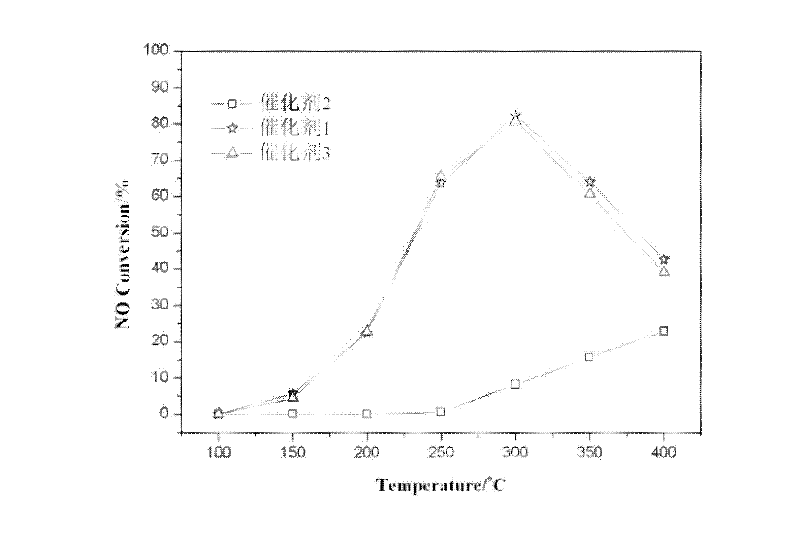
[0052] Take by weighing 2.22g manganese acetate tetrahydrate and dissolve to obtain the aqueous solution of manganese, take by weighing 5.00g titanium dioxide (TiO 2 ) into it, continuously stirred for 5 hours, 70°C rotary evaporation, 110°C overnight drying, 400°C roasting for 5h, after cooling, grinding, tableting, sieving, select 40-60 mesh catalyst for standby, denote as Catalyst 1. The mass of manganese acetate tetrahydrate was changed to 0.22g and 4.46g, and the amount of titanium dioxide was not changed. Under the same circumstances, the impregnated samples with different manganese loadings were recorded as catalyst 2 and catalyst 3, respectively.
Embodiment 2
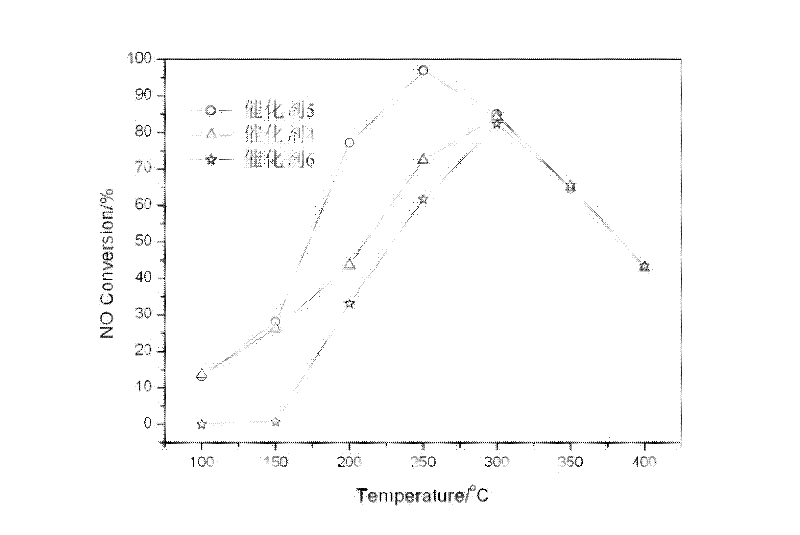
[0054] Take by weighing 14.32g mass fraction and be 50% manganese nitrate (Mn(NO 3 ) 2 ) solution plus deionized water to obtain Mn(NO 3 ) 2 solution, weighed 11.65g cobalt nitrate (Co(NO 3 ) 2 ) was dissolved in deionized water to obtain Co(NO 3 ) 2 Solution, according to the cobalt-manganese molar ratio is 1: 1 ratio mixing, adds the sodium carbonate of 0.5mol / L (Na 2 CO 3 ) and sodium hydroxide (NaOH) mixture (molar ratio Na 2 CO 3 / NaOH=1:2), until pH=10, make Mn 2+ and Co 2+ The metal ions are completely precipitated, continue to stir the suspension for 5 hours, age for 12 hours, filter out the resulting precipitate, wash with deionized water, dry at 110°C overnight, roast at 300°C in air atmosphere for 5 hours, grind, tablet, sieve, select The 40-60 mesh catalyst is reserved as catalyst 4. Using cerium (Ce) and copper (Cu) instead of cobalt (Co) as the second active component, catalyst 5 and catalyst 6 were prepared in the same way.
Embodiment 3
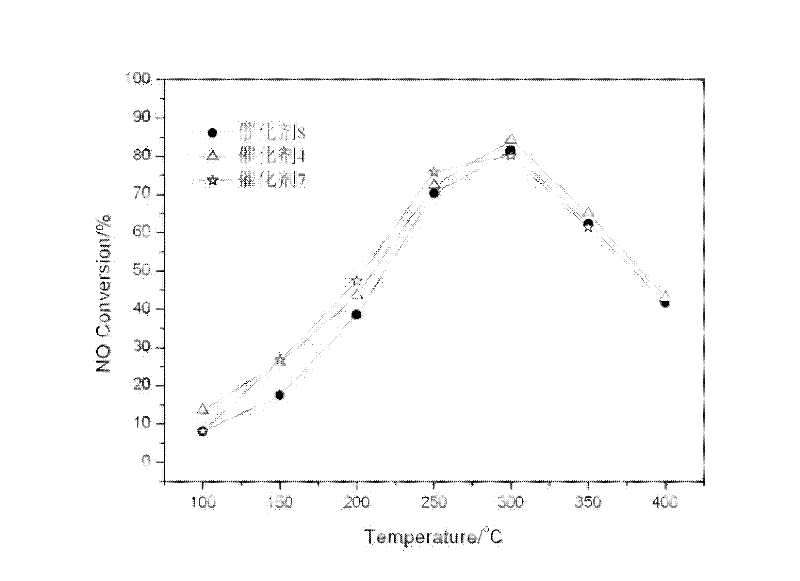
[0056] Take by weighing 14.32g mass fraction and be 50% manganese nitrate (Mn(NO 3 ) 2 ) solution plus deionized water to obtain Mn(NO 3 ) 2 solution, weighed 23.30g cobalt nitrate (Co(NO 3 ) 2 ) was dissolved in deionized water to obtain Co(NO 3 ) 2 Solution, mix according to the ratio of cobalt-manganese molar ratio of 2: 1, add 0.5mol / L sodium carbonate (Na 2 CO 3 ) and sodium hydroxide (NaOH) mixture (molar ratio Na 2 CO 3 / NaOH=1:2), until pH=8, make Mn 2+ and Co 2+ The metal ions are completely precipitated, continue to stir the suspension for 10 hours, age for 24 hours, filter the resulting precipitate, wash with deionized water, dry at 110°C overnight, roast at 500°C for 3 hours in an air atmosphere, grind, press, sieve, and select The 40-60 mesh catalyst is reserved, and is recorded as catalyst 7. Change the consumption of cobalt nitrate, weigh 5.83g cobalt nitrate, the consumption of manganese nitrate is unchanged. Under the same circumstances, a catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com