Novel HIV recombined multi-epitope fusion antigen and application thereof
A recombinant antigen and multi-epitope technology, applied in the field of molecular biology and immunology, can solve the problem of clinical value to be evaluated, and achieve the effect of reducing non-specific adsorption, improving efficiency, and not easy to miss detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
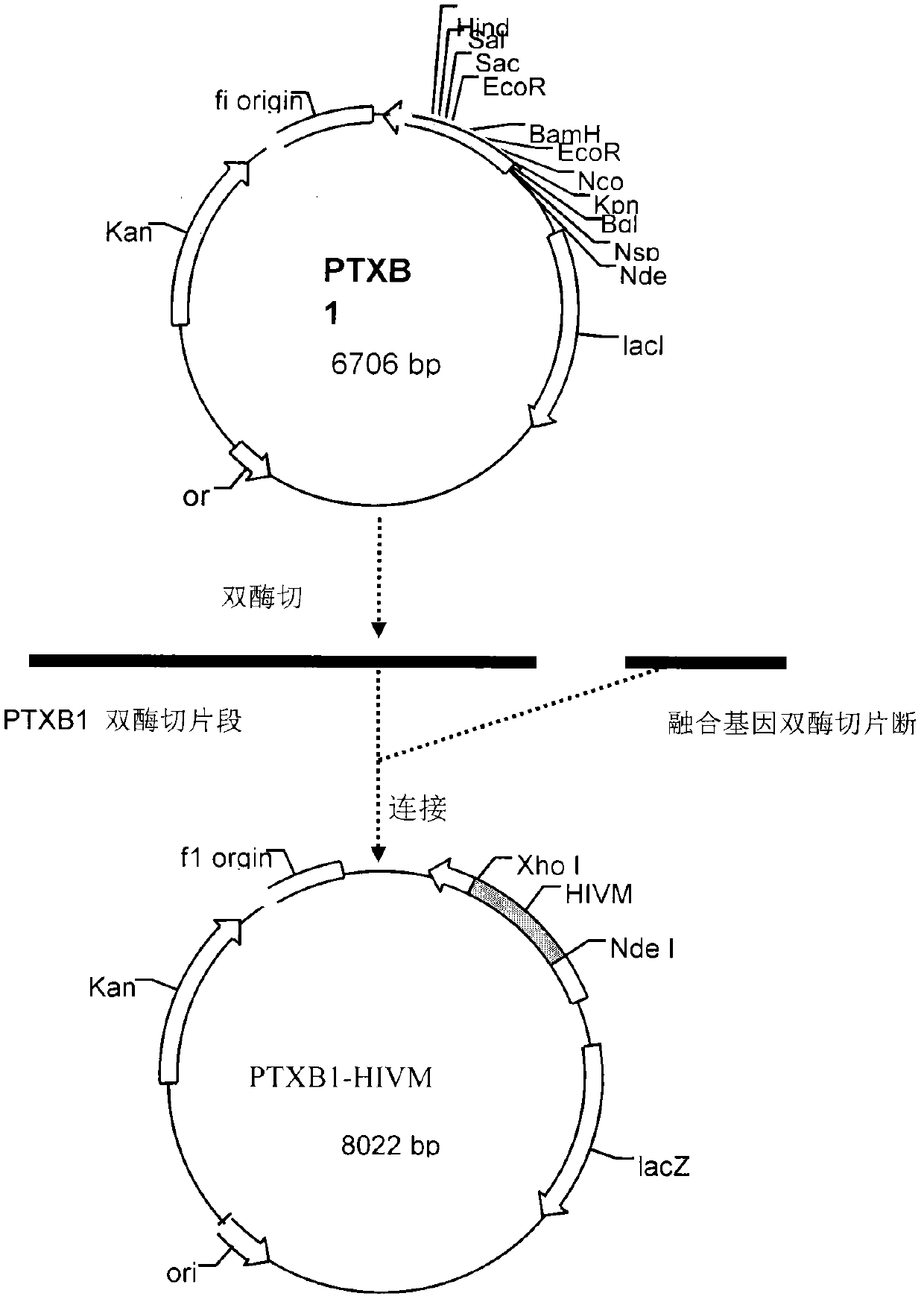
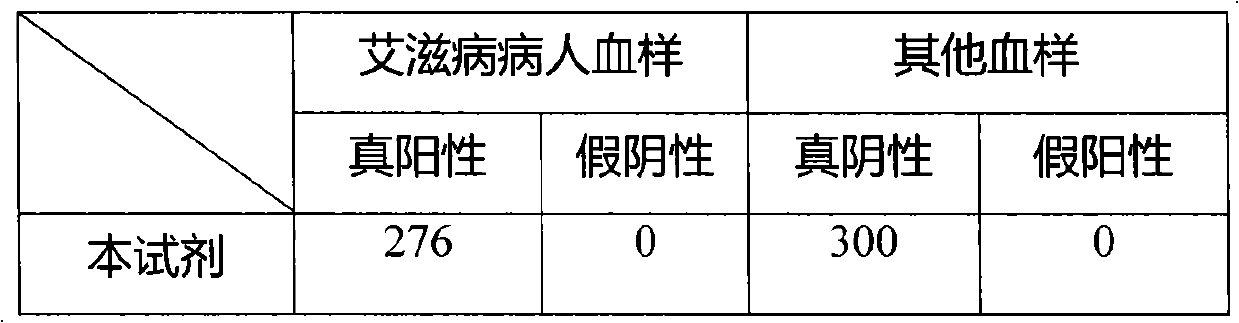
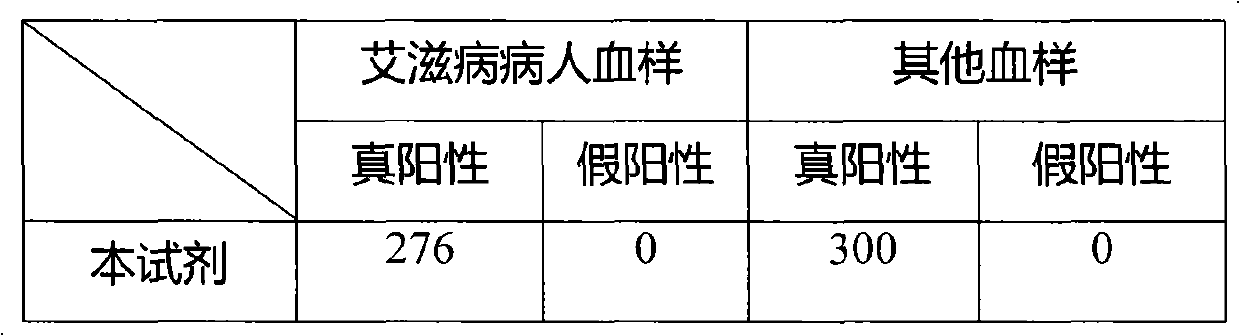
Image
Examples
Embodiment 1
[0043] Application of Recombinant Fusion Protein in HIV ELISA Detection
[0044] 1. Preparation of enzyme-linked plate coated with recombinant antigen: Dilute pTXB1HIVM recombinant antigen with 0.05M, pH9.6 carbonic acid buffer, preferably at a concentration of 2ug / ml, add 100ul / well to the enzyme-linked plate, and keep at 4°C for 24 hours. Discard the liquid, add blocking solution at 200ul / well, and keep at 4°C for 24 hours. Discard the liquid, dry at room temperature less than 30% humidity for 24 hours, and store in vacuum for later use. The blocking solution is 0.02M phosphate buffer containing 0.5% Casein, 10% calf serum, 2% sucrose and 0.1% proclin-300.
[0045] 2. Enzyme conjugate preparation: HRP was labeled with pTXB1HIVM, and the optimal concentration of the enzyme conjugate was selected by conventional square titration method.
[0046] 3. Preparation of quality control serum: collect HIV positive serum, mix more than 5 parts, measure its OD value, finally dilute to...
Embodiment 2
[0058] Application of recombinant fusion protein in the detection of HIV gold standard test strips
[0059] 1 Preparation of coating membrane: 0.02M pH 7.2 phosphate buffer solution is preferred as the coating solution. After filtering through a 0.22um membrane, store it at 4°C for later use, and the validity period is one week. Debug the film spraying machine (Bio-Dot), the preferred film spray volume is 20ul / 30cm, dilute recombinant pTXB1HIVM antigen with coating buffer, the concentration is 0.5mg / ml, dilute the prepared "sheep anti-pTXB1HIVM" with coating buffer "Polyantibody" to a concentration of 0.5mg / ml, machine scribing, the distance between the two lines is 5mm, it should be fine and uniform, and dry at room temperature for 20 minutes. Post-dry at 37°C for 2 hours, seal the bag and prepare the test cardboard for pasting.
[0060] 2 Preparation of the composite pad: 1200ml of deionized water is placed in a clean glass vessel (siliconization is possible if possible), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com