Method for determining content of azithromycin in azithromycin sustained-release eye drops
A technology for slow-release eye drops and azithromycin, which is applied in the field of content determination of azithromycin in Chinese medicine, can solve the problems of low recovery rate, determination of drug content, leakage of the main drug, etc., and achieve time and cost reduction, accurate detection data, and specificity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Mobile phase: methanol-acetonitrile-0.02mol dipotassium hydrogen phosphate buffer (adjust pH=7.2 with phosphoric acid)=50:10:40;
[0045] Determination steps:
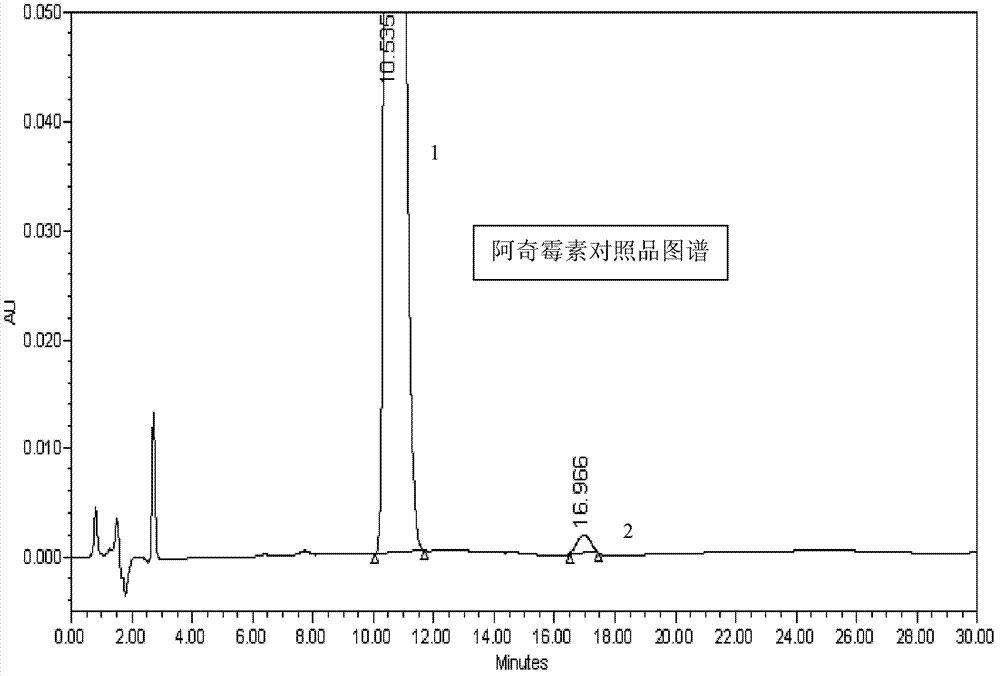
[0046]Weigh 5g of azithromycin sustained-release eye drops sample, place it in a 25ml volumetric flask, add mobile phase to half of the volumetric flask, shake gently, and set the volume to the mark. Weigh 50 mg of azithromycin reference substance, place it in a 25ml volumetric flask, add mobile phase to dissolve the reference substance and dilute to the mark with mobile phase. Take the reference substance solution and the sample solution respectively and pass through a 0.22 μm filter membrane, carry out high-performance liquid chromatography analysis according to the above conditions, and record the chromatogram. The obtained chromatogram shows that under this condition, the main peak of azithromycin can be completely separated from the auxiliary material peak. According to reference substance concentration / s...
Embodiment 2
[0048] Mobile phase: methanol-acetonitrile-0.02mol disodium hydrogen phosphate buffer volume ratio (adjust pH=7.2 with phosphoric acid)=20:50:30;
[0049] Determination steps:
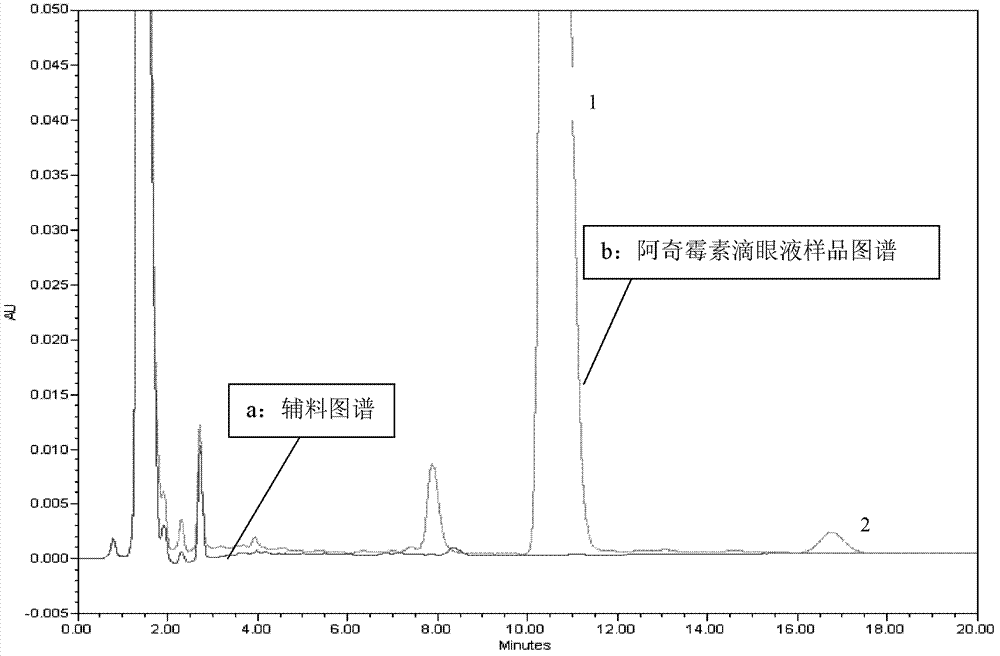
[0050] Quantitatively weigh 5g of azithromycin sustained-release eye drops sample, place it in a 25ml volumetric flask, add mobile phase to half of the volumetric flask, shake gently, and set the volume to the mark. Weigh 50mg of azithromycin reference substance, place it in a 25ml volumetric flask, add mobile phase to dissolve and dilute to the mark. Take the reference substance solution and the sample solution respectively and pass through a 0.22 μm filter membrane to carry out high-performance liquid chromatography analysis according to the above conditions, and record the chromatograms. The obtained chromatograms are shown in the attached figure 1 As shown, it can be seen that the azithromycin main peak 1 can be completely separated from the auxiliary material peak under this condition, and the az...
Embodiment 3
[0053] Mobile phase: methanol-acetonitrile-0.02mol dipotassium hydrogen phosphate buffer volume ratio (adjust pH=6.8 with phosphoric acid)=60:20:20;
[0054] Determination steps:
[0055] Quantitatively weigh 5g of azithromycin sustained-release eye drops sample, place it in a 25ml volumetric flask, add mobile phase to half of the volumetric flask, shake gently, and set the volume to the mark. Weigh 50mg of azithromycin reference substance, place it in a 25ml volumetric flask, add mobile phase to dissolve and dilute to the mark. The reference substance solution and the sample solution were respectively passed through a 0.22 μm filter membrane and analyzed by high performance liquid chromatography according to the above conditions, and the obtained spectrum showed that the main peak of azithromycin could be completely separated from the peak of the auxiliary material under this condition. According to reference substance concentration / sample concentration=contrast peak area / sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com