Biphenyl compound, synthetic method and application thereof
A synthesis method and compound technology, which is applied in the field of biphenyl compounds and their synthesis, can solve the problems of high cost, and achieve the effects of cost saving, shortening process flow, and green and clean production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
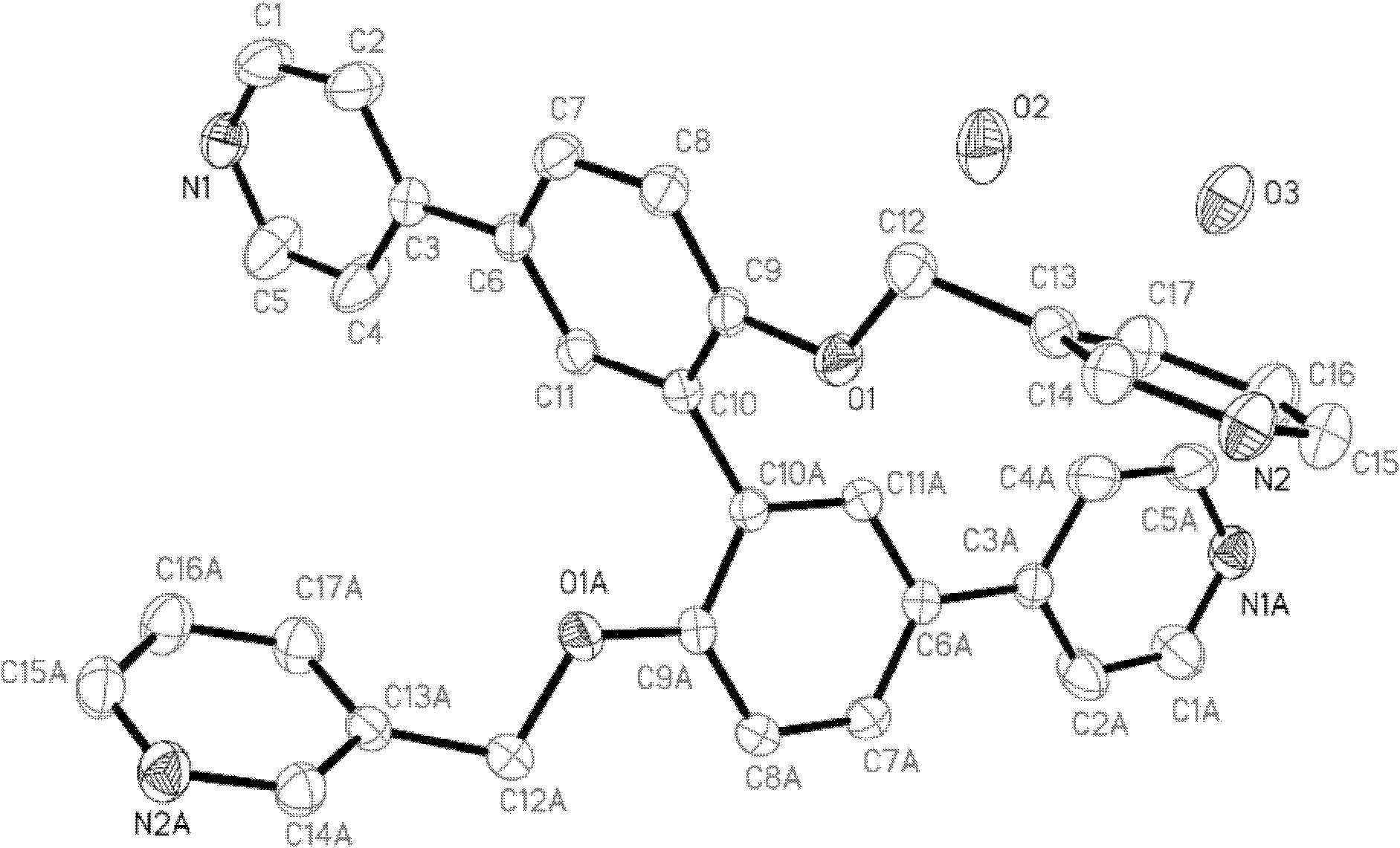
[0039] Embodiment 1: the preparation of catalyst
[0040] 1.5,5'-dibromo-2,2'-diphenol (3.44g, 10mmol), 3-chloromethylpyridine hydrochloride (3.80g, 25mmol), K 2 CO 3 (27.6g, 200mmol), KI (0.60g, 3.6mmol), DMF 25mL, stirred at room temperature for 36h, the reactant was poured into water, suction filtered, and the filter cake was subjected to silica gel column chromatography (CH 2 Cl 2 :CH 3 OH=25:1), to obtain 4.27 g of white solid intermediate B with a yield of 812%.
[0041] 2.N 2 Under protection, intermediate B (5.26g, 10mmol), pyridine-4-boronic acid (3.08g, 25mmol), K 2 CO 3 (17.25g, 125mmol), placed in a 250mL three-necked flask, toluene, ethanol and water as solvent, adding catalyst Pd (PPh 3 ) 4 (1.2g, 1mmol), heat to reflux, control the temperature at 90°C-110°C, and control the reflux time at 24h. TLC tracking, left standstill after reaction finishes, cooling, liquid separation, spin-dried organic phase, silica gel column chromatography (CH 2 Cl 2 :CH 3O...
Embodiment 2
[0042] Embodiment 2: the preparation of catalyst
[0043] 1.5,5'-dibromo-2,2'-diphenol (3.44g, 10mmol), 3-chloromethylpyridine hydrochloride (3.04g, 20mmol), K 2 CO 3 (20.7g, 150mmol), KI (0.80g, 4.8mmol), DMF 25mL, stirred at room temperature for 24h, the reactant was poured into water, suction filtered, and the filter cake was subjected to silica gel column chromatography (CH 2 Cl 2 :CH 3 OH=25:1), to obtain white solid intermediate B4.12g, yield 78.3%.
[0044] 2.N 2 Under protection, intermediate B (5.26g, 10mmol), pyridine-4-boronic acid (2.46g, 20mmol), K 2 CO 3 (20.7g, 150mmol), placed in a 250mL three-necked flask, toluene, ethanol and water as solvent, adding catalyst Pd (PPh 3 ) 4 (1.2g, 1mmol), heat to reflux, control the temperature at 90°C-110°C, and control the reflux time at 36h. TLC tracking, left standstill after reaction finishes, cooling, liquid separation, spin-dried organic phase, silica gel column chromatography (CH 2 Cl 2 :CH 3 OH=20:1), to o...
Embodiment 3
[0045] Embodiment 3: Cu(NO 3 ) 2 Oxidation of water under hydrothermal to produce hydrogen peroxide
[0046] With catalyst ligand L 5.22mg (0.01mmol) prepared due to embodiment 1, Cu(NO 3 ) 2 ·6H 2 O 12.0mg (0.04mmol), water 2mL, sealed in a 10cm glass tube, programmed to heat up to 150°C after 300min, keep the temperature for 4320min, and then drop to 30°C after 3000min to obtain a yellow monovalent copper salt polymer and A solution containing hydrogen peroxide.
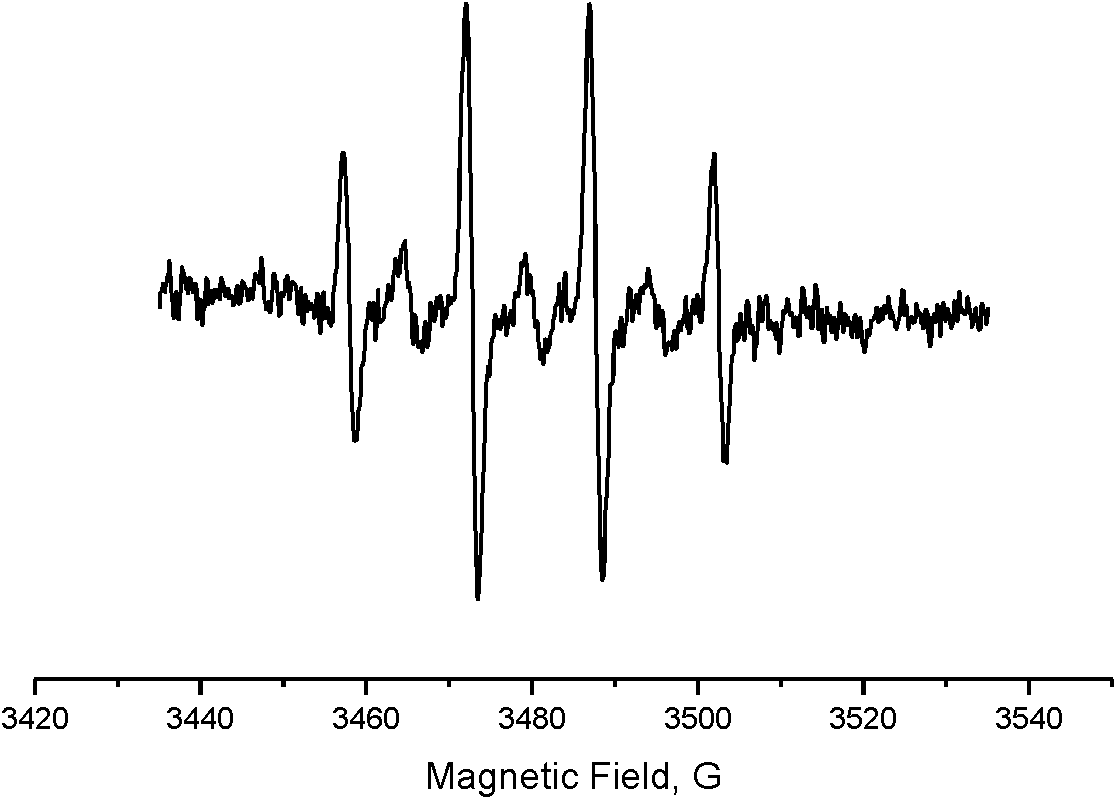
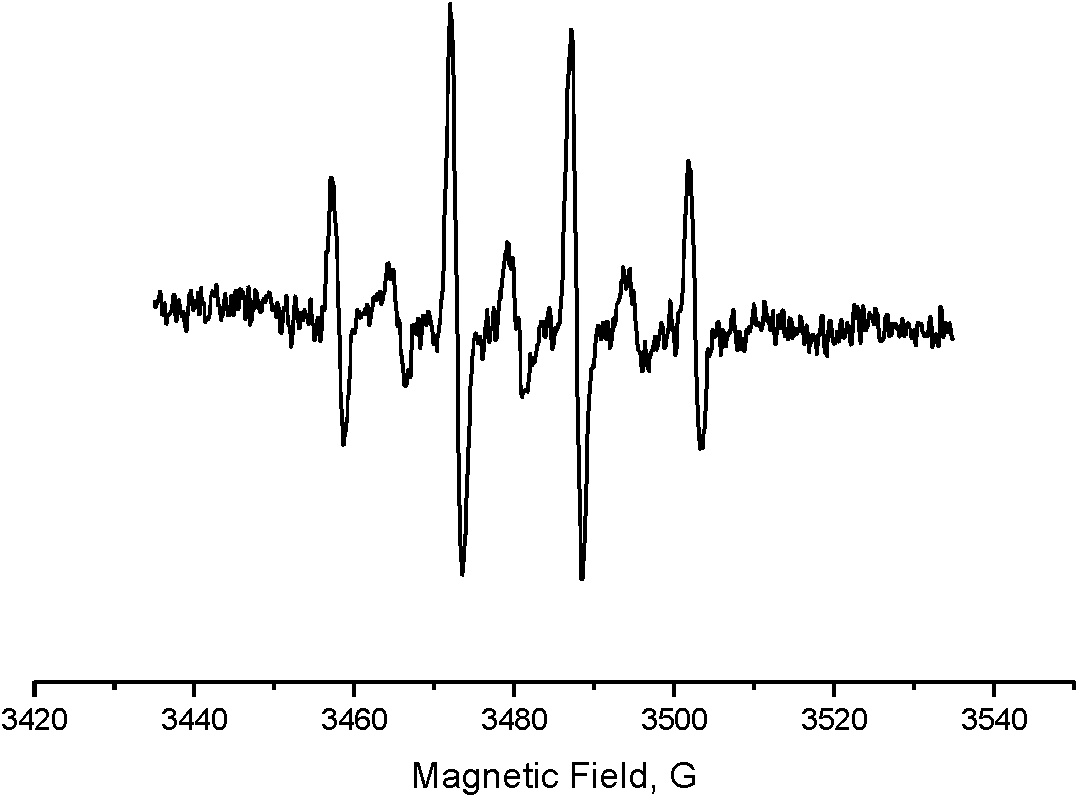
[0047] image 3 as a catalyst with Cu(NO 3 ) 2 The solution obtained by catalytic oxidation of water is added to the EPR signal measured by the hydroxyl radical scavenger DMPO, thus it can be concluded that the water is oxidized to hydrogen peroxide and partially produces hydroxyl radicals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com