Waste alkali solution recovery method
A recovery method and alkali solution technology, which is applied in the field of waste alkali solution recovery, can solve problems such as cellulose removal that cannot meet the recycling standard, and achieve the effects of alleviating the difficulty of filtration, improving utilization, and reducing COD and BOD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
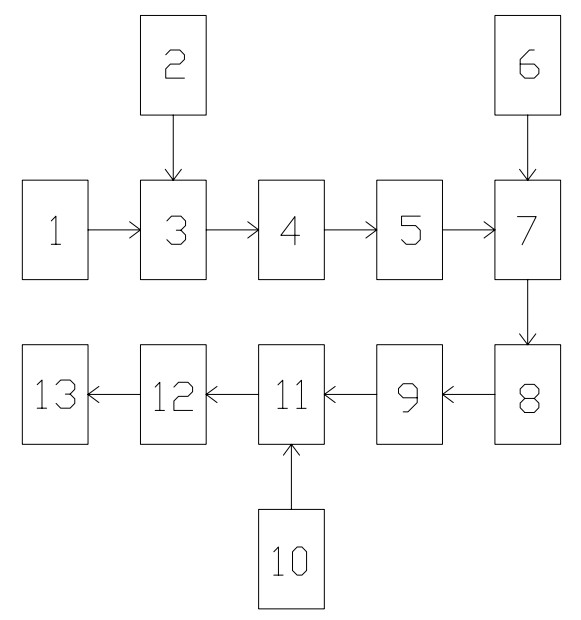
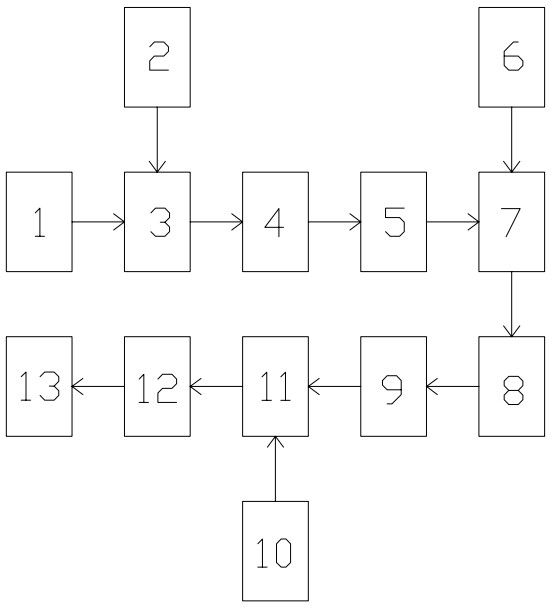
[0043] A recovery method for spent alkali solution, comprising the following process steps:
[0044] A, primary coagulation: add inorganic polymer coagulant aqueous solution and organic polymer coagulant aqueous solution successively to spent caustic soda solution, and stir simultaneously;
[0045] B. Primary precipitation filtration: perform a precipitation filtration on the spent alkali solution after primary coagulation;
[0046] C. Secondary coagulation: Add an aqueous solution of an inorganic polymer coagulant and an aqueous solution of an organic polymer coagulant to the spent caustic soda solution after the primary precipitation and filtration, and stir simultaneously;
[0047] D. Secondary precipitation filtration: perform secondary precipitation filtration on the spent alkali solution after secondary coagulation;
[0048] E. Oxidation: Oxidize the waste alkali solution after secondary precipitation and filtration by using an oxidant;
[0049] F. Adsorption: Activate...
Embodiment 2
[0053] On the basis of Example 1, wherein the oxidation process of step E is preferably:
[0054] The oxidizing agent is one of ozone, hydrogen peroxide, chlorine dioxide, oxygen (air), sodium hypochlorite, potassium permanganate, and nano-titanium dioxide, or a plurality of them in any proportion.
[0055] Wherein the oxidizing agent is preferably the combination of ozone and hydrogen peroxide, ozone (O 3 ) and hydrogen peroxide (H 2 o 2 ) in a molar ratio of 1:0.25;
[0056] The specific oxidation process is as follows: Ozone is introduced into the waste alkali solution after secondary precipitation and filtration, and hydrogen peroxide is sprayed at the same time, and the molar ratio of added ozone and hydrogen peroxide is kept at 1:0.25, and the treatment is carried out for 10 minutes until the secondary precipitation and filtration The spent caustic solution is colorless and clear.
Embodiment 3
[0058] On the basis of Example 1, wherein the oxidation process of step E is preferably:
[0059] The oxidizing agent is one of ozone, hydrogen peroxide, chlorine dioxide, oxygen (air), sodium hypochlorite, potassium permanganate, and nano-titanium dioxide, or a plurality of them in any proportion.
[0060] Wherein the oxidizing agent is preferably the combination of ozone and hydrogen peroxide, ozone (O 3 ) and hydrogen peroxide (H 2 o 2 ) in a molar ratio of 1:2.5;
[0061] The specific oxidation process is as follows: Ozone is introduced into the spent alkali solution after secondary precipitation and filtration, and hydrogen peroxide is sprayed at the same time, and the molar ratio of added ozone and hydrogen peroxide is kept at 1:2.5, and the treatment is carried out for 25 minutes until the secondary precipitation and filtration The spent caustic solution is colorless and clear.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com