Benzopyrrolidiketone-based semiconductor polymer and preparation and purpose thereof
A technology of diketopyrrole and diketopyrrole, which is applied in the field of diketopyrrole-based semiconducting polymers and its preparation, can solve problems such as difficulty in large-scale application, and achieve low cost and low energy synthesis. Band gap, simple and effective synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
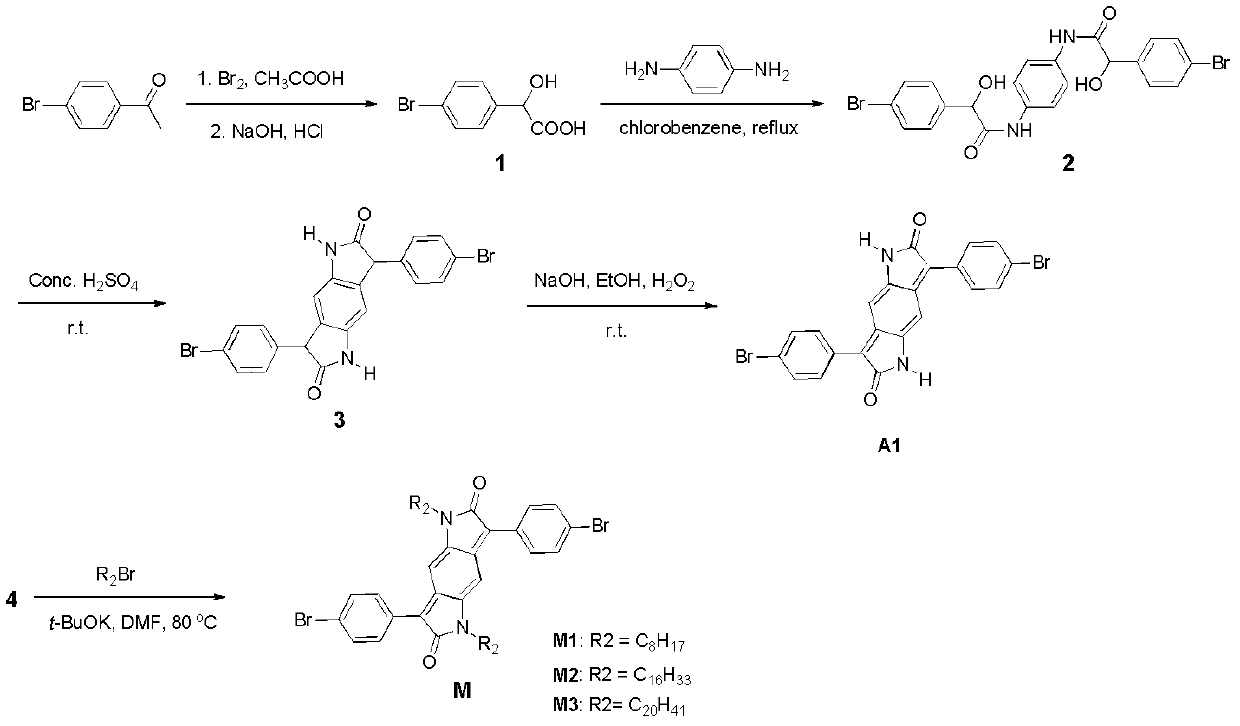
[0034] Embodiment 1, preparation of the semiconducting polymer of benzopyrrole diketope group
[0035] This example provides three kinds of soluble benzopyrrole diketope-based semiconducting polymers, the structural formula of which is shown in Table 1 (wherein, n≥1), and its synthetic route can be found in figure 1 .
[0036] Table 1
[0037]
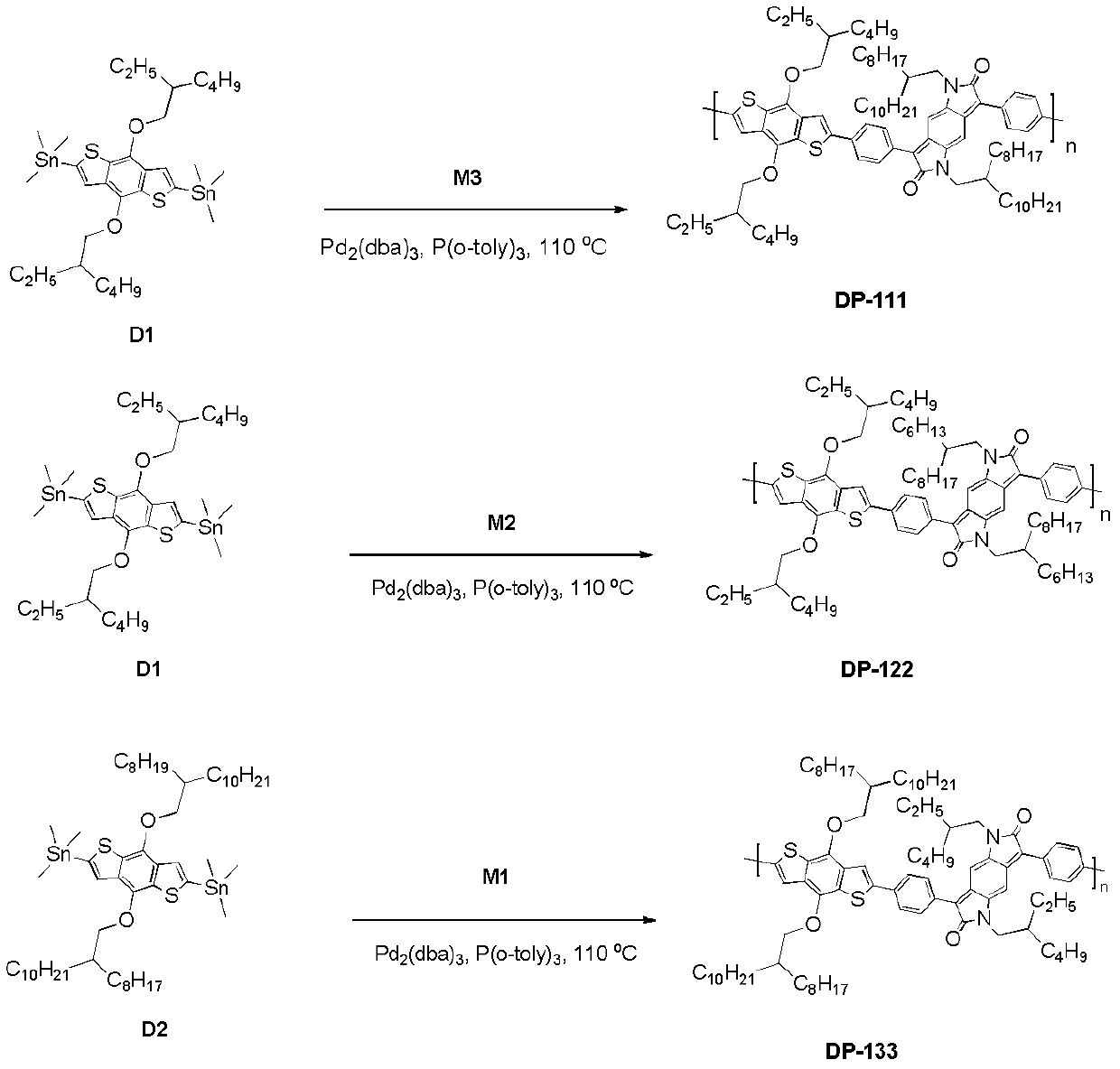
[0038] 1.1. Preparation of compounds DP-111, DP-122 and DP-133
[0039] The preparation method of the polymer (DP-111, DP-122 and DP-133) containing benzopyrrole diketopyl group comprises the steps:
[0040] (a) Synthesis of Intermediate Compound A
[0041] The structural formula of intermediate compound A is
[0042] For its detailed preparation method, see the document "Greenhalgh, C.W., Carey, J.L. and Newton, D.F., the synthesis of quinodimethanes in the benzodifuranone anf benzodipyrrolidone series, Dyes Pigm., 1, 103-120, (1980)".
[0043] (b) Synthesis of compounds M1, M2 and M3
[0044] The structural formulas of...
Embodiment 2
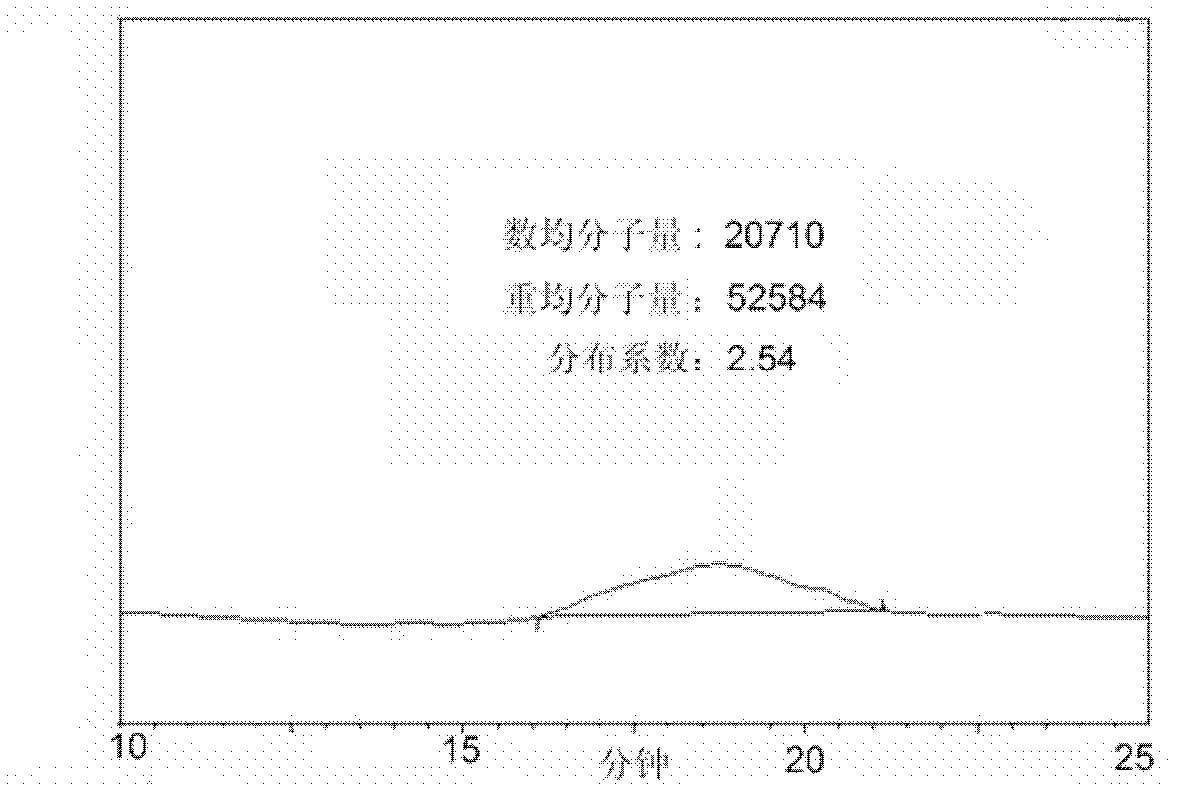
[0051] Gel Permeation Chromatography, UV Absorption Spectrum and Electrochemical Properties of Embodiment 2, Polymer DP-133
[0052] image 3 The number average molecular weight measured by gel permeation chromatography (GPC) of the polymer is 20710, and the distribution coefficient PDI is 2.54. Figure 4 The ultraviolet absorption spectrum of polymer DP-133 in chloroform is given, its maximum absorption peak is around 614nm, and its optical band gap is 1.57eV. Figure 5 The cyclic voltammetry curve of compound DP-133 is given. The cyclic voltammetry test is carried out on the computer-controlled CHI610D telephone line analyzer, using the traditional three-electrode test system, the platinum electrode is the working electrode, the silver / silver ion electrode is the reference electrode, and the electrolyte is tetra-n-butyl hexafluorophosphoric acid Ammonium dichloromethane solution (0.1M), the scanning speed is 50mv / s, with ferrocene as a reference. The oxidation potential...
Embodiment 3
[0053] Embodiment 3, polymer DP-133 as the purposes in thin-film solar cell as hole transport material
[0054] Figure 7 A schematic structural diagram of a bulk heterojunction organic thin film solar cell device is given, which uses polymer DP-133 as hole transport material and P71BM as acceptor. Solar cell device test I-V curve see Figure 8 , the conversion efficiency data are shown in Table 2: the conversion efficiency of the preliminary test is 0.2%, and the device shows a high open circuit voltage (0.90V).
[0055] Table 2 The parameter data of the thin film solar cell device with DP-133 / PC71BM as the semiconductor layer
[0056]
[0057] In summary, the benzopyrrole diketope-based semiconducting polymers involved in the present invention are characterized in that they have a large π-conjugated main chain body with a rigid plane and flexible solubilized alkyl side chains, and have a low energy band gap, low HOMO energy level, and thin-film solar cell device test...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
| distribution coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com