Method for preparing carbonyl compounds by alcohol catalytic oxidation through oxygen without transition metal
A technology of transition metal catalysis and carbonyl compounds, which is applied in the field of carbonyl compound preparation, can solve the problems of low catalytic activity of secondary alcohols, and achieve the effects of easy operation control, mild reaction conditions, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
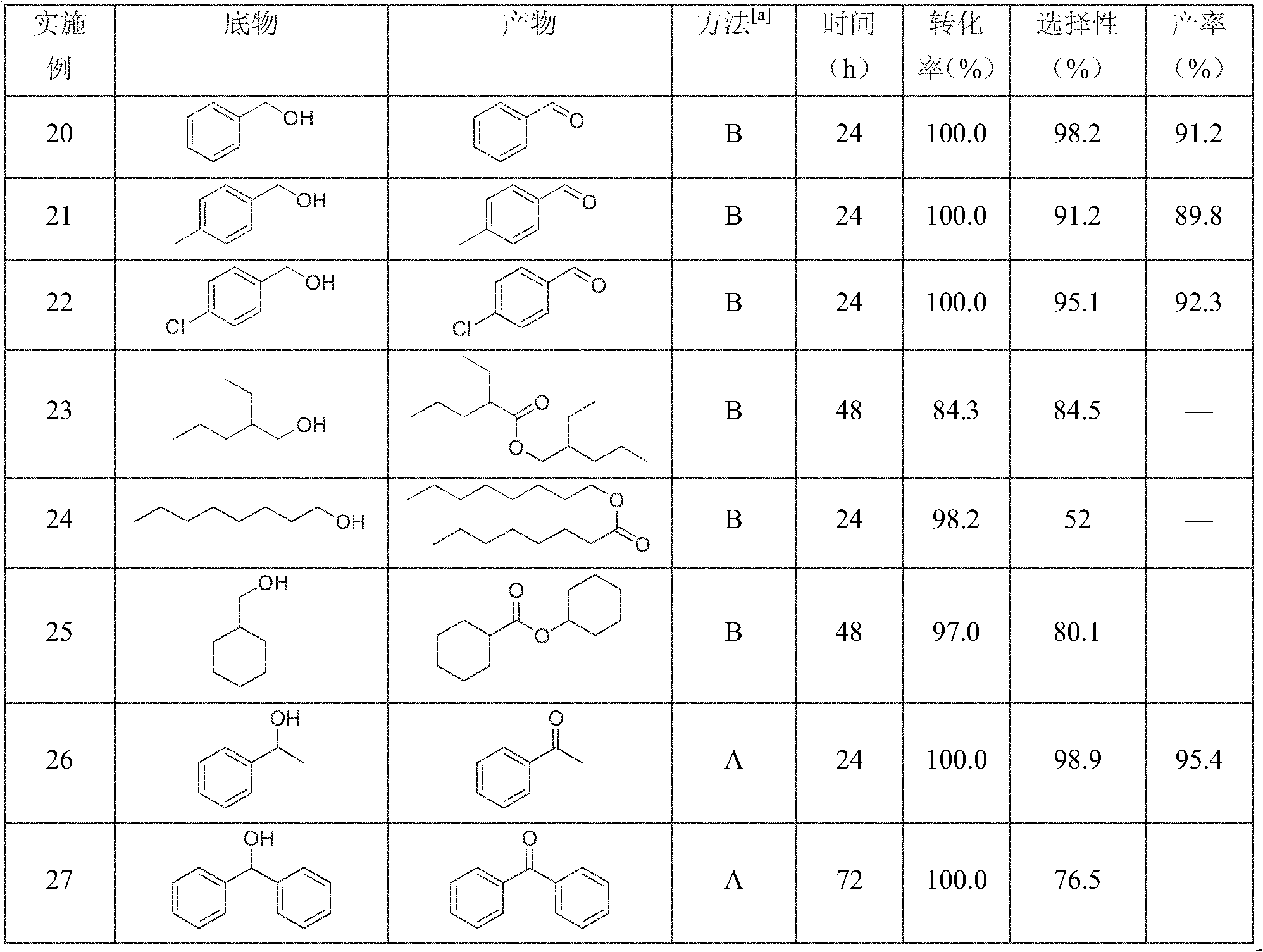
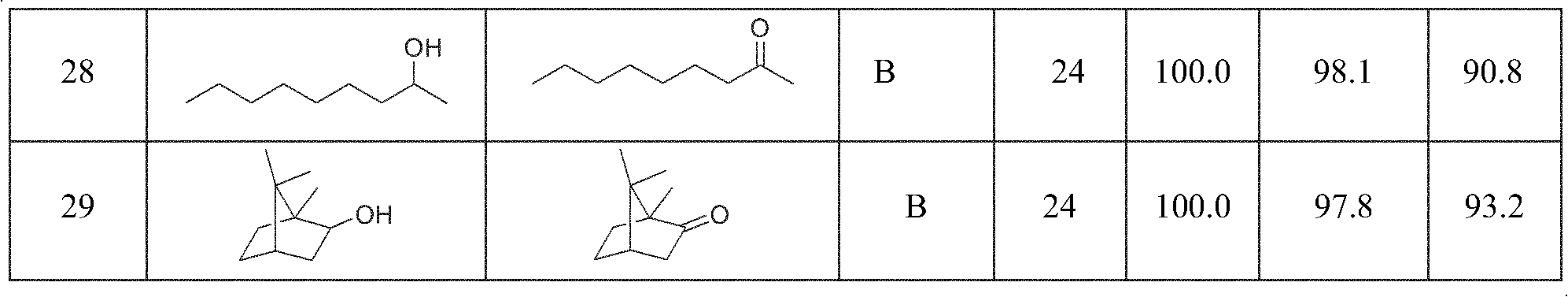
Examples
Embodiment 1
[0025] The oxidation reaction was carried out in a 25mL single-necked eggplant-shaped flask equipped with a stirring bar. Add 5.0mmol 1-phenylethanol, 0.25mmol N-bromosuccinimide (NBS), 0.25mmol tert-butyl nitrite (TBN), and 5mL ethyl acetate (EtOAc) to the eggplant-shaped bottle in turn, seal it and The top of the flask was directly connected to an oxygen-filled balloon. Heated and stirred in a water bath at 25°C, and samples were taken for gas chromatography to follow up and analyze the conversion rate and selectivity of the reaction, and the test samples were not purified. After the reaction was over, the stirring was stopped. The reaction liquid was transferred to a separatory funnel, then the eggplant flask was carefully washed with EtOAc, and the organic liquids were combined. followed by saturated Na 2 S 2 o 3 Aqueous solution and NaHCO 3 The organic phase was washed with aqueous solution to remove NBS and TBN. The organic layer was dried with anhydrous sodium su...
Embodiment 2
[0027] Test method and step are with embodiment 1, with 0.125mmol liquid bromine (Br 2 ) to replace NBS, the results are shown in Table 1.
Embodiment 3
[0029] The test method and steps are the same as in Example 1, with 0.25 mmol hydrobromic acid (HBr) replacing NBS, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com