Method for purifying capsular polysaccharide from capsular bacterium fermentation liquid
A technology of bacterial fermentation and capsular polysaccharide, applied in antibacterial drugs, bacterial antigen components, pharmaceutical formulations, etc., can solve problems such as increasing operation steps and operation time, substances that cannot be directly degraded, human and environmental hazards, etc. The effect of reducing protein and nucleic acid content, saving time and production costs, and improving recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
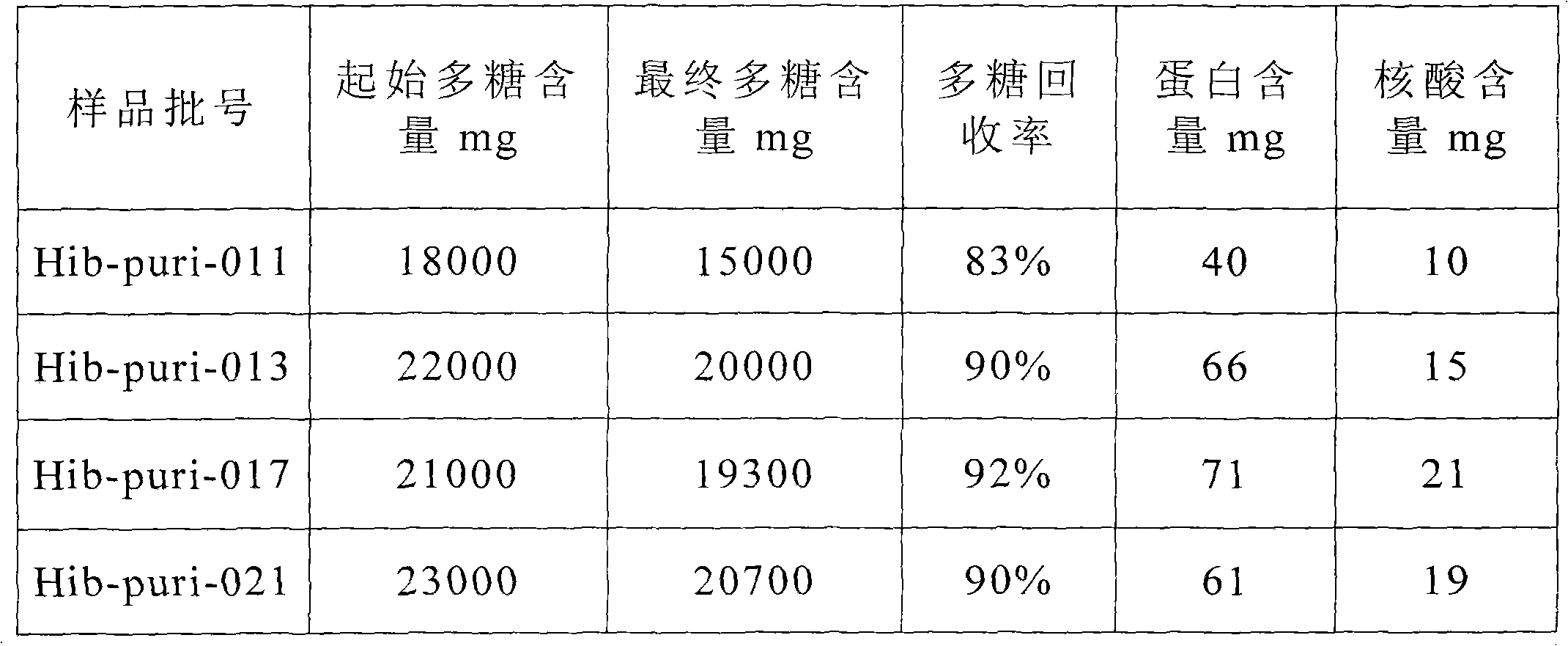
[0027] Example 1: Purification of capsular polysaccharide from Haemophilus influenzae type B fermentation broth
[0028] 1. Strain: Hib strain was purchased from ATCC, the strain number is 51654;
[0029] 2. Medium: MWSM I medium, MWSM II medium, MCDM I medium;
[0030] 3. Bacterial culture:
[0031] After melting the dry powder of the bacteria with the corresponding medium, spread it on the chocolate blood plate, and cultivate it overnight in the carbon dioxide incubator, scrape off the bacteria, put it into the first-level seed shaker flask and cultivate it for 4 hours, and then put the bacteria solution into the Put it into a 50L fermenter and cultivate it for 16 hours, and finally use formaldehyde to inactivate it.
[0032] 4. Purification of capsular polysaccharide:
[0033] Concentrate by centrifugation and ultrafiltration after fermented liquid volume is concentrated 10 times, add sodium acetate to make its mass percentage concentration be 1%, adjust pH value to be 4...
Embodiment 2
[0048] Example 2: Purification of capsular polysaccharide from N. meningitidis type A fermentation broth
[0049] 1. Strain: Neisseria meningitidis type A strain was purchased from China National Institutes for Food and Drug Control, strain number CMCC 29201;
[0050] 2. Medium: MWSM I medium, MWSM II medium, MCDM I medium;
[0051] 3. Bacterial culture:
[0052] After melting the dry powder of the bacteria with the corresponding medium, spread it on the chocolate blood plate, and cultivate it overnight in the carbon dioxide incubator, scrape off the bacteria, put it into the first-level seed shaker flask and cultivate it for 4 hours, and then put the bacteria solution into the Put it into a 50L fermenter and cultivate it for 16 hours, and finally use formaldehyde to inactivate it.
[0053] 4. Purification of capsular polysaccharide:
[0054] Concentrate the fermented liquid volume by centrifugation and ultrafiltration to 30 times, add sodium acetate to make the mass percent ...
Embodiment 3
[0069] Example 3: Purification of capsular polysaccharide from pneumococcal fermentation broth
[0070] 1. Strains: Pneumococcus strains were purchased from China National Institutes for Food and Drug Control, strain number CMCC31218;
[0071] 2. Medium: MWSM I medium, MWSM II medium, MCDM I medium;
[0072] 3. Bacterial culture:
[0073] After melting the dry powder of the bacteria with the corresponding medium, spread it on the chocolate blood plate, and cultivate it overnight in the carbon dioxide incubator, scrape off the bacteria, put it into the first-level seed shaker flask and cultivate it for 4 hours, and then put the bacteria solution into the Put it into a 50L fermenter and cultivate it for 16 hours, and finally use formaldehyde to inactivate it.
[0074] 4. Purification of capsular polysaccharide:
[0075] Concentrate by centrifugation and ultrafiltration after fermented liquid volume is concentrated 20 times, add sodium acetate to make its mass percentage conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com