Quinoline-substituted anhydride naphthalene derivative, iridium complex thereof and application in pH (potential of hydrogen) sensing
A technology of iridium complexes and derivatives, applied in the field of sensors, can solve the problems of single emission color change, narrow pH value detection range, inaccurate pH value, etc., and achieve the effects of fast response speed, high sensitivity and simple preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of 6-(4-phenyl-quinolin-2-yl)-benzo[de]isochromene-1,3-diketone (PQBD)
[0046] Dissolve 2.62 g (20 mmol) of aluminum trichloride into 20 mL of DCM, add 1.54 g (10 mmol) of acenaphthylene after the complete dissolution of aluminum trichloride, then add 2 mL of acetic anhydride, and stir the solution at room temperature for 3 hours , then poured into a large amount of water, stirred until the color of the solution turned pale yellow, then extracted with DCM, dried with anhydrous magnesium sulfate and concentrated, and used PE:EA (20:1, v:v) as the eluent column Chromatographic separation gave a yellow product, which was finally recrystallized from ethanol to give 0.137 g of light yellow crystal 1-acenaphthyl-5-ylethanone. Yield: 70%. 1 H NMR (CDCl 3 , 400 MHz) δ (ppm): 8.74-8.72 (d, 1H), 8.08-8.07 (d, 1H), 7.62-7.58 (t, 1H), 7.38-7.36 (d, 1H), 7.31-7.30 (d , 1H), 3.36-3.35 (m, 4H), 2.56-2.55 (m, 3H). GC-MS (m / z): 196.
[0047] Take 0.1 g (0...
Embodiment 2
[0050] Embodiment 2: Complex (PQBD) 2 Preparation of Ir(pic)
[0051] Weigh IrCl 3 ·3H 2 O (0.352 g, 1 mmol) was added to a three-necked flask, vacuum-filled with nitrogen-vacuumized on the double-row tube, and cycled three times, and finally the reaction system was protected with nitrogen. 6-(4-Phenyl-quinolin-2-yl)-benzo[de]isochromene-1,3-dione (PQBD) (1.001 g, 2.5 mmol), poured into water (3 mL, 165 mmol) Add ethylene glycol ether (9mL, 93mmol) into the reaction system, stir, and raise the temperature of the reaction system to 120°C. The reaction time is 24 hours, and a reddish-brown precipitate is formed during the reaction. The reaction system was cooled to room temperature, and then the precipitate was filtered and washed with water and ethanol to obtain a reddish-brown solid product, namely the iridium dichloro bridge compound of PQBD.
[0052] Weigh the iridium dichloro bridge compound (0.411g, 0.2 mmol) and Na of PQBD 2 CO 3(1.0 mmol) and added to a three-necke...
Embodiment 3
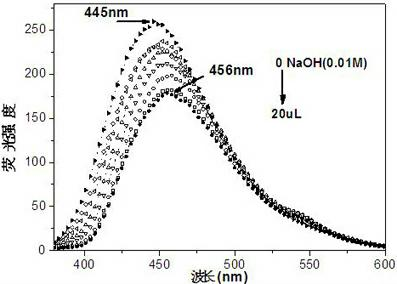
[0054] Embodiment 3: The ultraviolet absorption of PQBD and the fluorescence emission spectrometry titration experiment of PQBD when the pH value is constantly increasing:
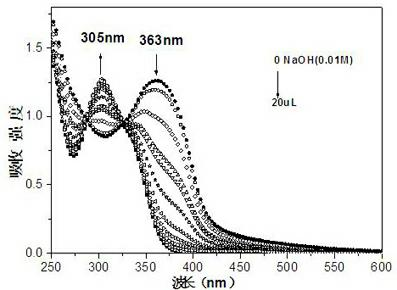
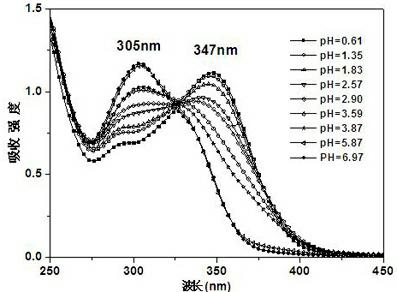
[0055] Compound PQBD was formulated as 4 × 10 -5 mol / L of DMSO-H 2 O (1:9, v / v) solution, pipette 2.5 mL of the prepared compound PQBD solution in a fluorescence cuvette, and drop 2 μL of 1×10 -2 mol / L NaOH aqueous solution until equilibrium is reached (that is, the absorption spectrum no longer changes significantly). When no NaOH aqueous solution was added dropwise, the absorption spectrum of PQBD had a strong absorption peak at 363nm. When adding constantly with NaOH aqueous solution, when pH value constantly increases, the absorption peak at 363nm place weakens gradually, appears new absorption peak at 305nm place, and the intensity of new peak constantly increases with the increase of pH value (as in the accompanying drawing figure 1 shown). The entire absorption spectrum has a blue shift, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com