Production method for high-purity magnesium fluoride crystal
A pure magnesium fluoride and production method technology, applied in the direction of magnesium fluoride, magnesium halide, etc., can solve the problems of low recycling efficiency and high environmental pressure, achieve good economic benefits, reduce environmental pressure, and reduce production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
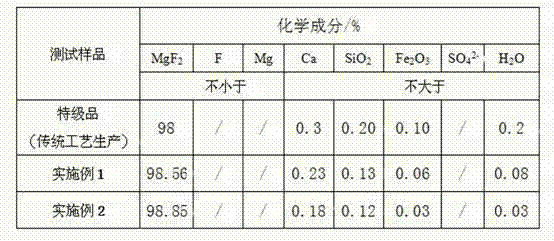
Examples
Embodiment 1
[0016] The production method of the high-purity magnesium fluoride crystal of the present embodiment comprises the following steps:
[0017] (1) Mix industrial-grade magnesium carbonate with water, stir, and prepare a suspension of magnesium carbonate with a concentration of 10% by weight, and introduce CO into the suspension of magnesium carbonate 2 gas, CO 2 The partial pressure of the gas is 0.2 atmospheric pressure, the carbonization reaction is carried out for 40 hours, and the reaction temperature is controlled to be 30° C., and then the reaction solution A is obtained. The reaction solution A is a mixed solution of magnesium carbonate and magnesium bicarbonate, and is set aside;
[0018] (2) Take fluosilicic acid, a by-product of the phosphate fertilizer industry, and ammonolyze it to obtain an ammonium fluoride solution with a concentration of 50% by weight;
[0019] (3) Mix the reaction solution A obtained in step (1) with the ammonium fluoride solution obtained in s...
Embodiment 2
[0022] The production method of the high-purity magnesium fluoride crystal of the present embodiment comprises the following steps:
[0023] (1) Mix high-purity magnesium carbonate with water, stir, and prepare a suspension of magnesium carbonate with a concentration of 1% by weight, and pass CO into the suspension of magnesium carbonate 2 gas, CO 2 The partial pressure of the gas is 10 atmospheres, the carbonization reaction is carried out for 2 hours, and the reaction temperature is controlled to be 1° C., and then the reaction solution A is obtained. The reaction solution A is a mixed solution of magnesium carbonate and magnesium bicarbonate, and is set aside;
[0024] (2) Take fluosilicic acid, a by-product in the production process of anhydrous hydrofluoric acid, and ammonolyze it to obtain an ammonium bifluoride solution with a concentration of 5% by weight;
[0025] (3) Mix the reaction solution A obtained in step (1) with the ammonium bifluoride solution obtained in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com