Process for preparing cyclohexanone and cyclohexanol by cyclohexane selective oxidation
A technology of cyclohexane and cyclohexanone, which is applied in the fields of catalytic chemistry, organic chemistry and industrial chemical synthesis, can solve the problems of low selectivity and harsh catalyst preparation, and achieves simple preparation process, good cycle stability, and preparation of raw materials. Inexpensive and accessible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
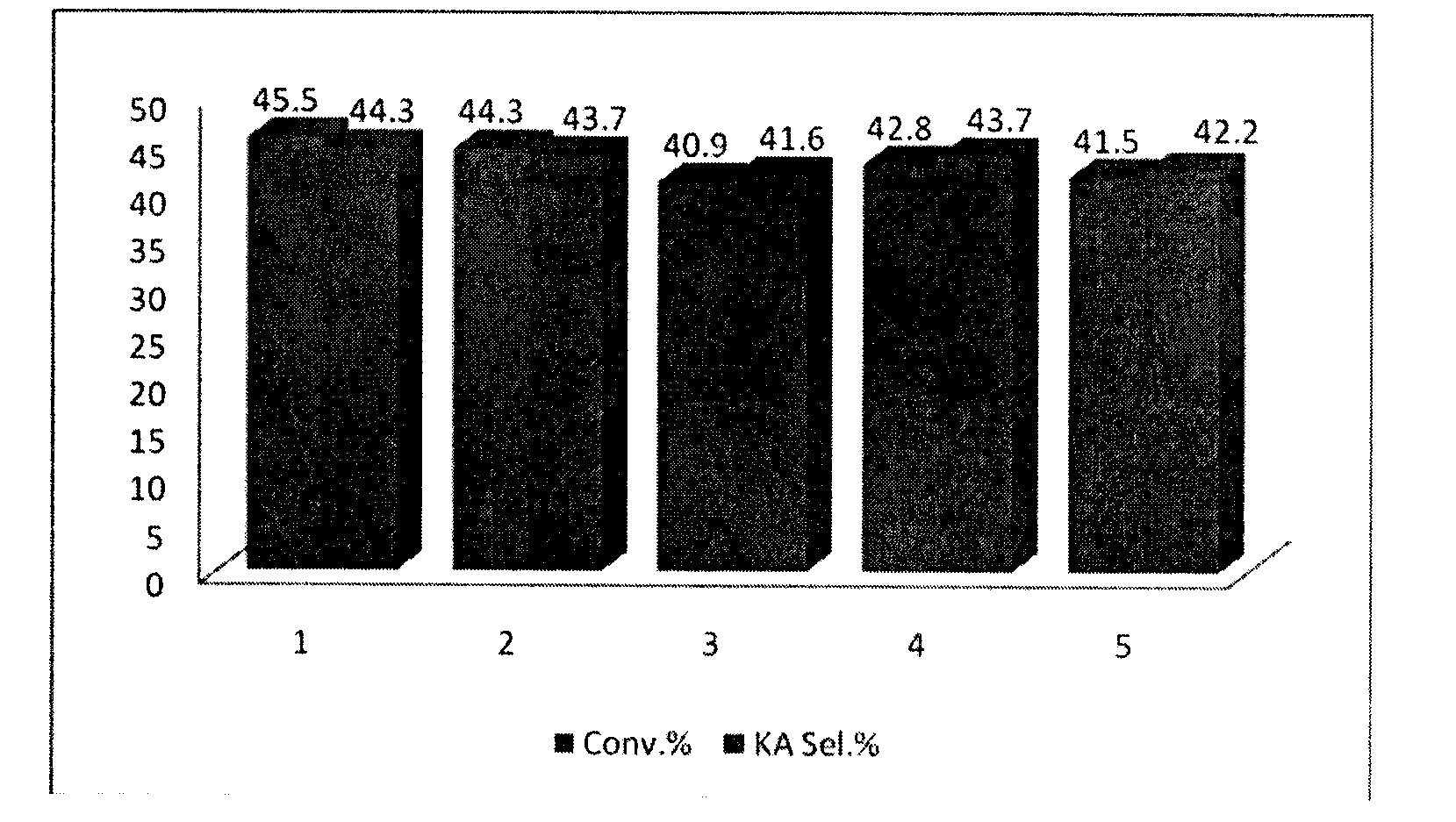
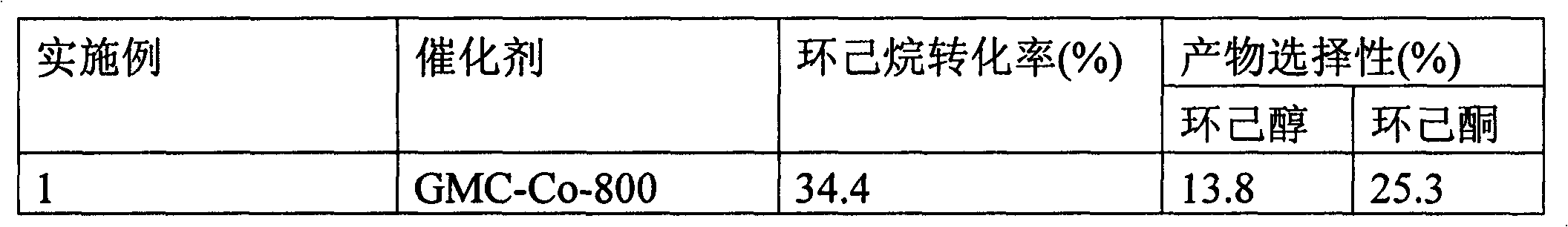
[0023] Embodiment 1-4 is to use phenol and furfural as carbon source with Co(NO 3 ) 2 Comparison of Catalytic Selective Oxidation of Cyclohexane to Cyclohexanone and Cyclohexanol Catalyzed by Graphitized Catalysts Obtained at Different Carbonization Temperatures. The experimental conditions are: 125ml stainless steel autoclave, 12g cyclohexane, 8g acetonitrile, 0.3g cyclohexanone, catalyst GMC-Co-800 (carbonization at 800°C, Example 1), GMC-Co-900 (Example 2) , GMC-Co-1000 (Example 3), GMC-Co-1100 (Example 4), 50mg, 1.5MPa O 2 , 140 ° C reaction 2 ~ 10h.
[0024] The comparative results of Examples 1-4 are shown in Table 1.
[0025] Table 1
[0026]
[0027]
Embodiment 5
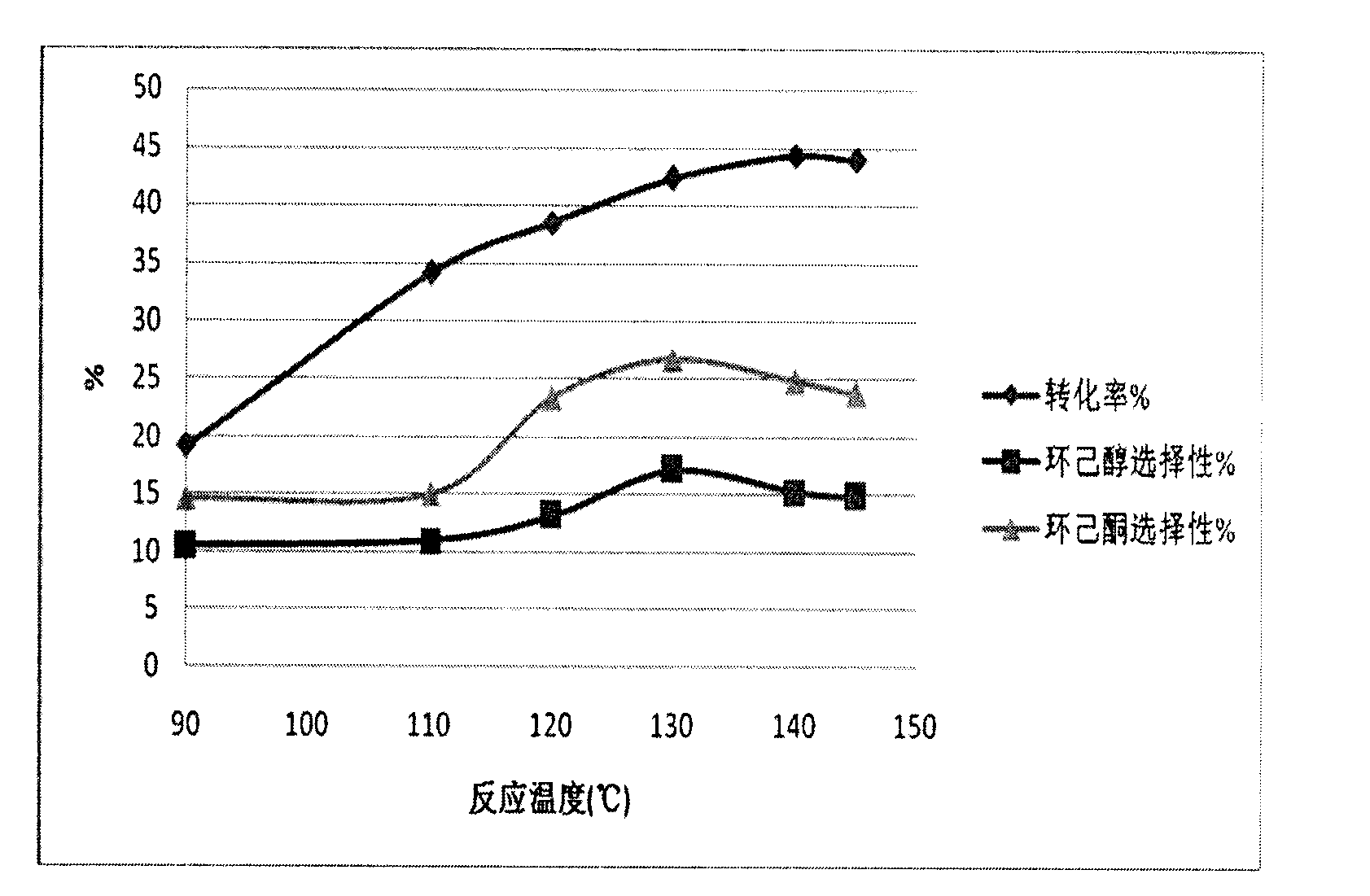
[0028] Embodiment 5-9 is with glucose and resorcinol as carbon source, with Fe(NO 3 ) 2 Comparison of Catalytic Selective Oxidation of Cyclohexane to Cyclohexanone and Cyclohexanol Catalyzed by Graphitized Catalysts. The experimental conditions are: 125ml stainless steel autoclave, 12g cyclohexane, 8g acetone, 0.3g cyclohexanone, catalyst GMC-Fe-2 (900°C carbonization for 2h, Example 5), GMC-Fe-4 (Example 6) , GMC-Fe-6 (Example 7), GMC-Fe-8 (Example 8), GMC-Fe-10 (Example 9), 100mg, 1.5MPa O 2 , 140 ° C reaction 2 ~ 10h. The comparison results of Examples 5-9 are shown in Table 2.
[0029] Table 2
[0030]
Embodiment 10
[0031] Embodiment 10-12 is with different carbon sources, with Fe(NO 3 ) 2 Comparison of Catalytic Selective Oxidation of Cyclohexane to Cyclohexanone and Cyclohexanol Catalyzed by Graphitized Catalysts. Experimental condition is: 125ml stainless steel autoclave 12g cyclohexane, 8g acetonitrile, 0.3g cyclohexanone, catalyst GMC-Fe-glucose / resorcinol (embodiment 10), GMC-Fe-sucrose / phloroglucinol ( Embodiment 11), GMC-Fe-furfural / phenol (embodiment 12), 50mg, 1.5MPa O 2 , 140 ° C reaction 2 ~ 10h. The comparative results of Examples 10-12 are shown in Table 3.
[0032] table 3
[0033]
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com