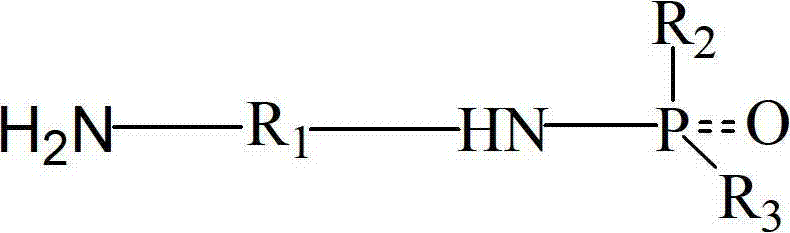Reaction type phosphorus-nitrogen fire retardant and synthesizing method thereof
A reactive flame retardant, phosphorus nitrogen flame retardant technology, applied in chemical instruments and methods, adhesive additives, non-polymer adhesive additives, etc., can solve poor dispersion, easy water absorption, and poor persistence of flame retardant effect and other problems, to achieve the effects of long-lasting flame retardant efficiency, simple preparation process, and little impact on performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
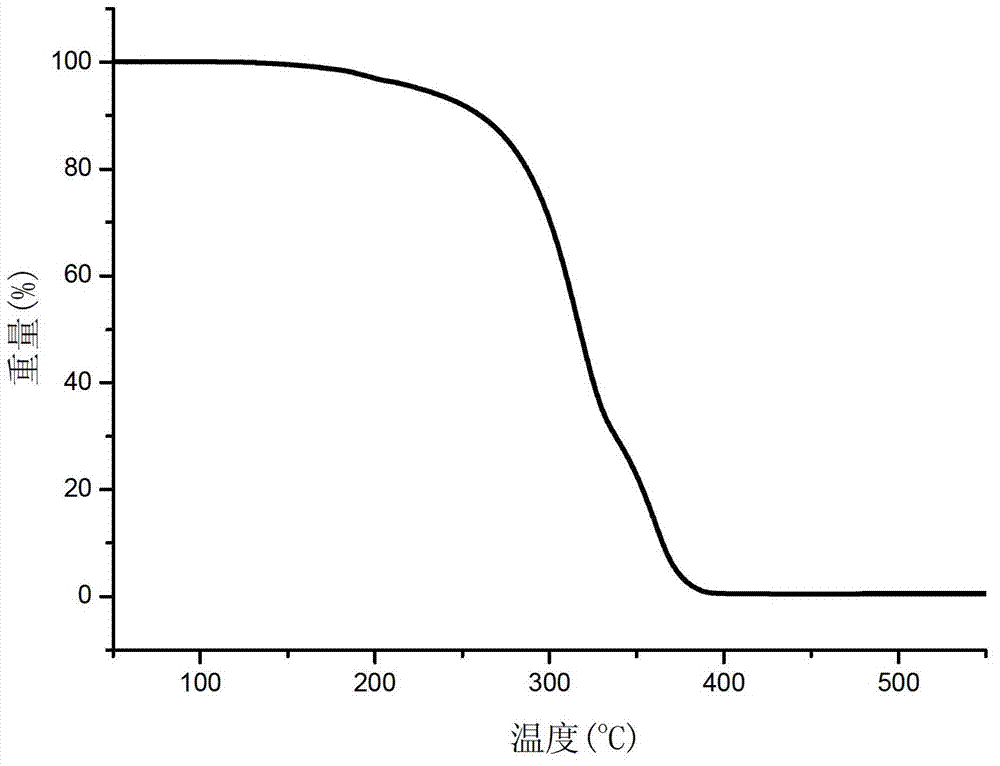
[0024] 6.01g (0.1mol) ethylenediamine, 15.2g (0.15mol) triethylamine and 80mL tetrahydrofuran were added to a 250mL four-necked flask and placed in an oil bath. Dry nitrogen was introduced at room temperature and the rate was 300 rpm. Stir continuously for 20 minutes at a rotation speed of 2 minutes; 22g (0.1mol) of diphenylphosphorus chloride was dissolved in 20mL of tetrahydrofuran, and then slowly dropped into the four-necked flask with a constant pressure dropping funnel. The dropping time was 2 hours. After the dropwise addition of phenylphosphonium chloride was completed, the temperature of the oil bath was increased to 75°C, and the reaction was continued with nitrogen for 6 hours. The obtained reaction product was filtered to obtain a filter cake, which was washed three times with deionized water, filtered, and filtered. The solid was dried at 120° C. for 5 hours and then ground and crushed to obtain 23.1 g of white product with a yield of 95%. The purity of the product ...
Embodiment 2
[0028] In a 500mL four-necked flask equipped with mechanical stirring and reflux condenser, 21.6g (0.2mol) o-phenylenediamine and 16.8g (0.3mol) potassium hydroxide were dissolved in 100mL petroleum ether, and nitrogen was introduced at room temperature. And continue to stir at 400 rpm for 30 minutes; 19.49g (0.1mol) phenylphosphoryl dichloride was dissolved in 50mL petroleum ether, and then slowly dropped into the four-necked flask with a constant pressure dropping funnel. The addition time is 2 hours. After the dropwise addition of phenylphosphoryl dichloride is completed, the temperature of the oil bath is increased to 65°C, and the reaction is continued with nitrogen for 7 hours. The obtained reaction product is filtered to obtain a filter cake, and the filter cake uses deionized water Wash three times, filter, dissolve the filtered solid in 50mL of DMF, filter, add a large amount of deionized water to the obtained filtrate, filter the precipitated solid, dry at 120°C for 5 ...
Embodiment 3
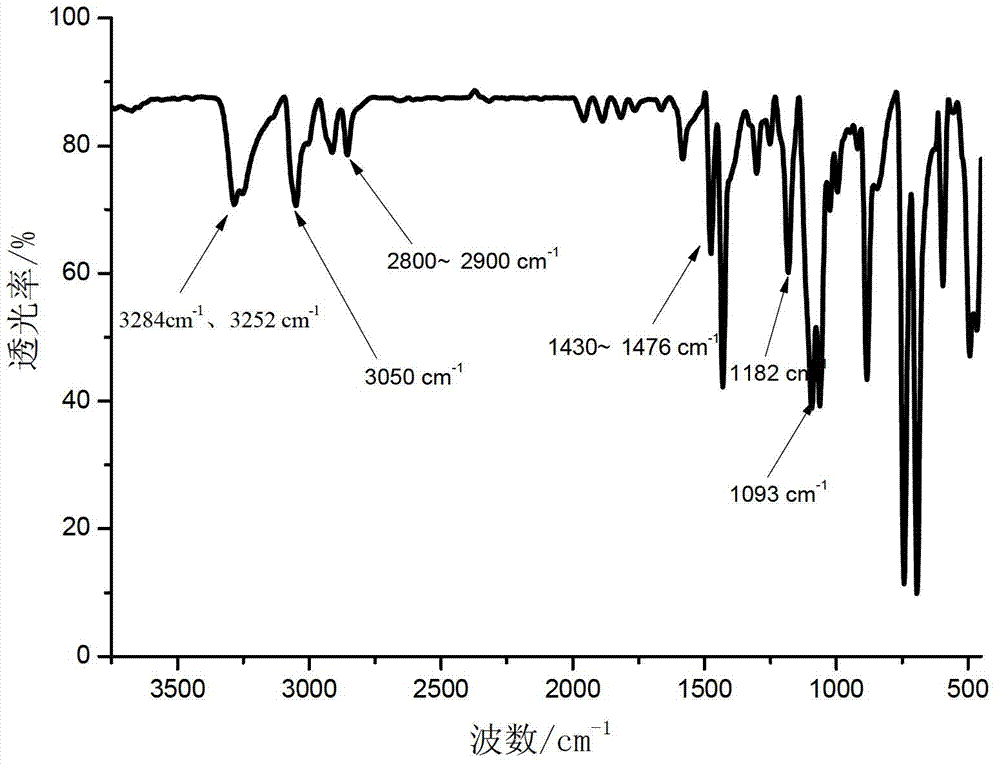
[0030] In a 500mL four-necked flask equipped with mechanical stirring and reflux condenser, 21.6g (0.2mol) m-phenylenediamine, 23.7g under normal temperature, stirring speed of 400 rpm and dry nitrogen (0.3mol) pyridine was dissolved in 100mL of toluene, 15.3g (0.1mol) of phosphorus oxychloride was dissolved in 30mL of toluene, and then slowly dripped into the four-necked flask with a constant pressure dropping funnel, the dripping time was 1.5 hours After the dropwise addition of phosphorus oxychloride is completed, the temperature of the oil bath is increased to 90°C, and the nitrogen gas is continued to react for 6 hours. The resulting reaction product is filtered to obtain a precipitate. The precipitated material is washed with deionized water, filtered, and filtered to obtain The solid was dried at 120°C for 5 hours to obtain 24.3 g of the final white solid product, the yield was about 94%, and the product purity was 99.5%. Infrared spectrum characterization: 3284cm -1 And...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com