Ternary copper catalyst CuO-Cu2O-Cu for synthesis of dimethyldichlorosilane and its preparation method
A technology of dimethyldichlorosilane and cuo-cu2o-cu, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of poor controllability of particle size and components, reducing agent Fe Problems such as difficult removal of powder and poor controllability of catalyst particle size, etc., achieve the effect of low price, high selectivity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take 10g of commercial CuO powder, put it into a fluidized bed reactor, and feed 200ml / min of N 2 , to make it fluidized; at 250°C, feed 7.5ml / min of CO gas, react for 120min, and crush and ball mill to obtain a ternary copper catalyst; the composition of the obtained copper catalyst after chemical analysis is: 4% Cu, 54% Cu 2 O and 42% CuO (weight percent, the same below).
[0034] The ternary copper catalyst material prepared above was subjected to an XRD test on an X'Pert PRO MPD multifunctional X-ray diffractometer produced by Panalytical Company of the Netherlands (Panalytical).
[0035] The particle size analysis of the ternary copper catalyst material prepared above was performed on a Dandong Baite BT-9300Z laser particle size distribution analyzer.
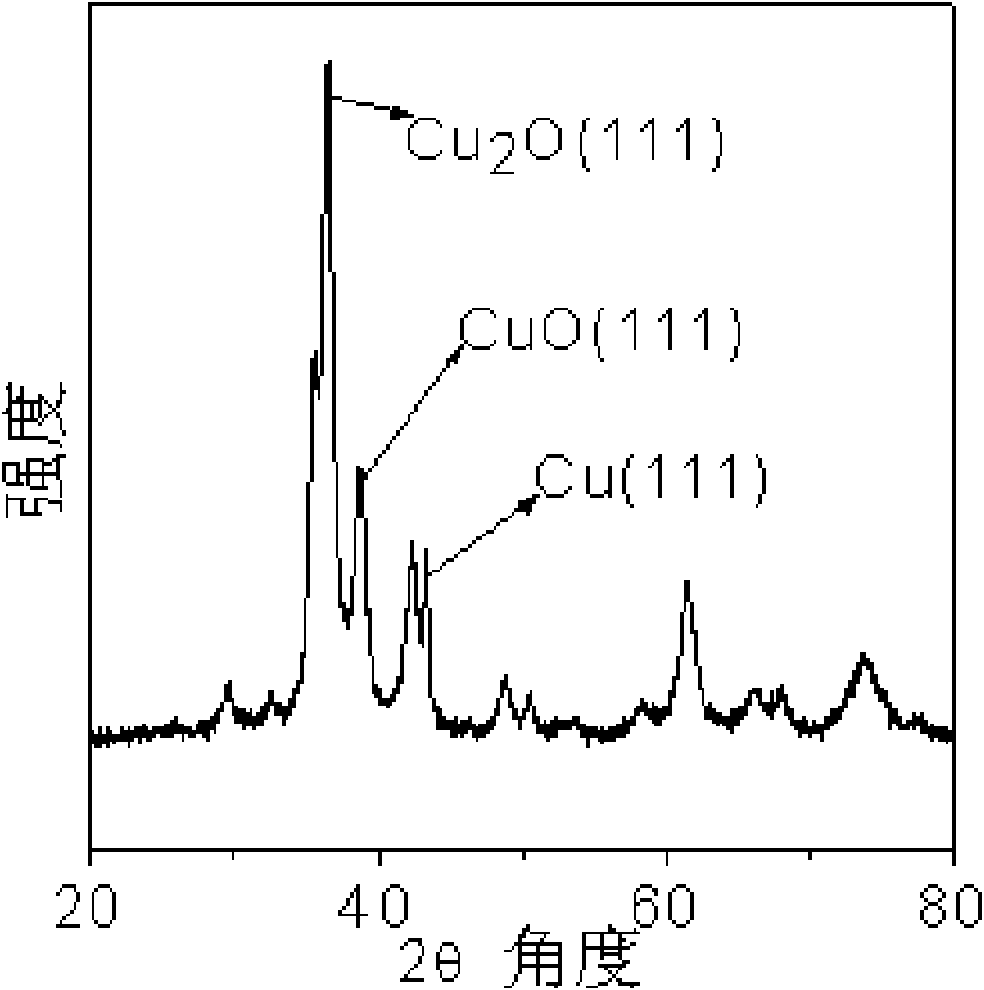
[0036] figure 1 It is the XRD pattern of the copper catalyst that embodiment 1 obtains, wherein 2θ=36.5 ° is Cu 2The characteristic peak of O, the shoulder peak that is made up of 2θ=35.5 ° and 2θ=38.8 ° is the ...
Embodiment 2
[0039] Take 10g of commercial CuO powder, put it into a stirred bed reactor, adjust the reaction temperature to 300°C, and feed 7.5ml / min of H 2 , reacted for 90min, and crushed and ball milled to obtain a ternary copper catalyst; the composition of the obtained copper catalyst was analyzed by chemical method: 3% Cu, 58% Cu 2 O, 38% CuO.
[0040] image 3 It is the XRD figure of the copper catalyst that embodiment 1 obtains, wherein 2θ=36.4 ° is Cu 2 The characteristic peak of O, the shoulder peak that is made up of 2θ=35.5 ° and 2θ=38.6 ° is the characteristic peak of CuO, and 2θ=43.2 ° is the characteristic peak of Cu, thus it can be seen that the catalyst prepared by this method is composed of Cu, Cu 2 A ternary copper catalyst composed of O and CuO.
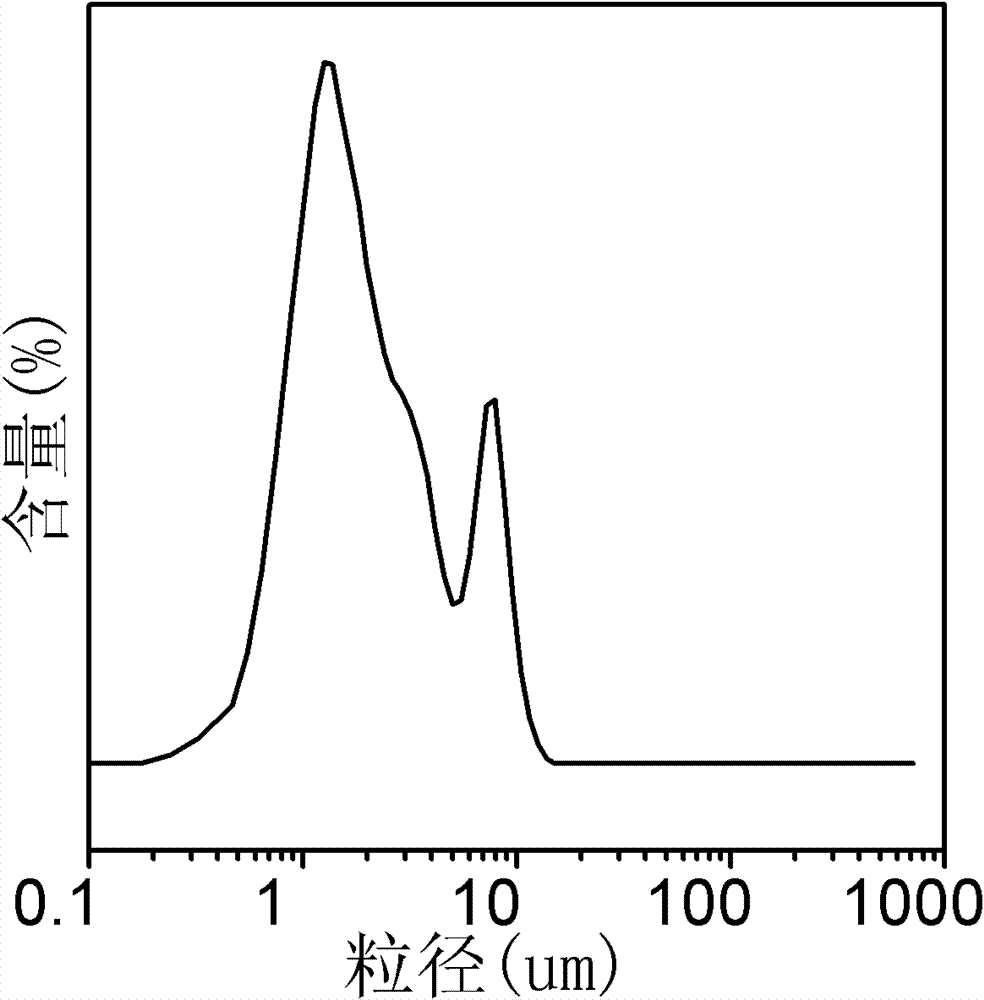
[0041] Figure 4 For the particle size analysis of the copper catalyst obtained in Example 1, it can be seen from the figure that the particle size of the copper catalyst prepared by this method is basically less than 10 ...
Embodiment 3
[0043] Take 10g of commercial CuO powder, put it into a fluidized bed reactor, adjust the reaction temperature to 200°C, and feed 200ml / min of N 2 , make it fluidized; adjust the CO gas flow to 6.7ml / min, the reaction time is 180min, and crush the ball mill to obtain the ternary copper catalyst; the obtained copper catalyst is composed of 7% Cu, 50% Cu after chemical analysis 2 O and 43% CuO.
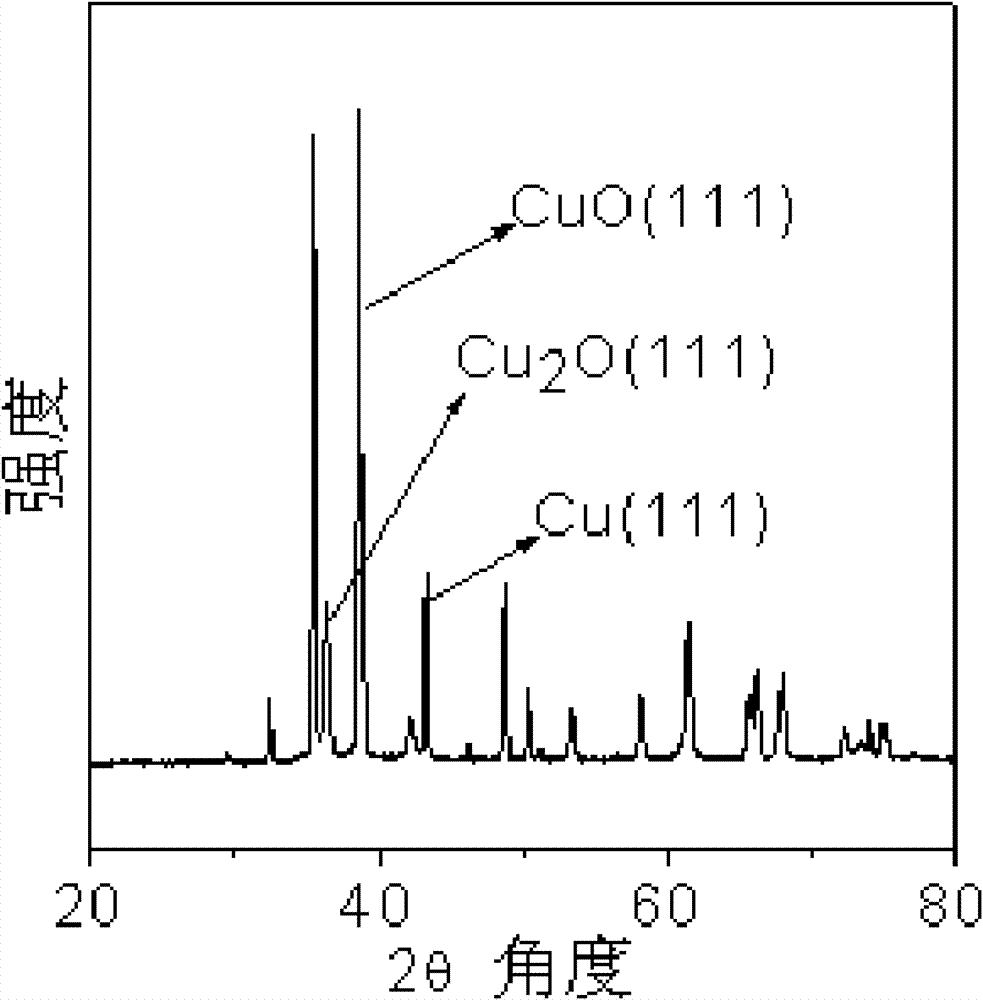
[0044] Figure 5 It is the XRD pattern of the copper catalyst that embodiment 3 obtains, wherein 2θ=36.6 ° is Cu 2 The characteristic peak of O, the shoulder peak that is made up of 2θ=35.5 ° and 2θ=38.8 ° is the characteristic peak of CuO, and 2θ=43.4 ° is the characteristic peak of Cu, thus it can be seen that the catalyst prepared by this method is composed of Cu, Cu 2 A ternary copper catalyst composed of O and CuO.
[0045] Figure 6 For the particle size analysis of the copper catalyst obtained in Example 3, it can be seen from the figure that the particle size of the copper c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com