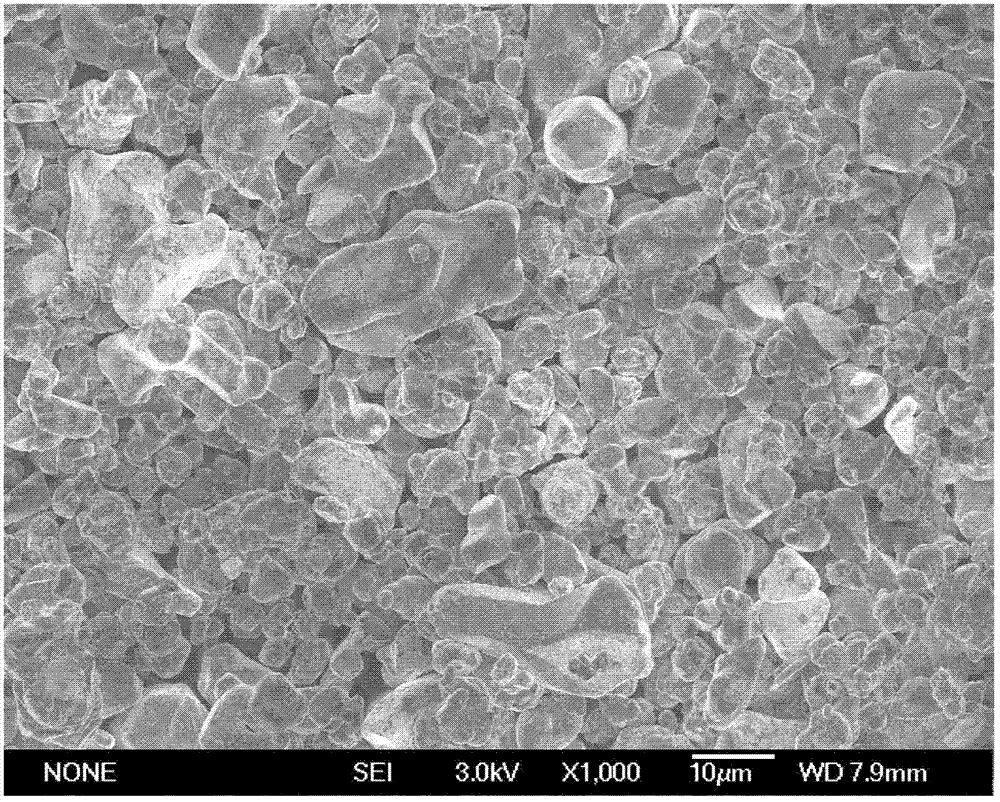
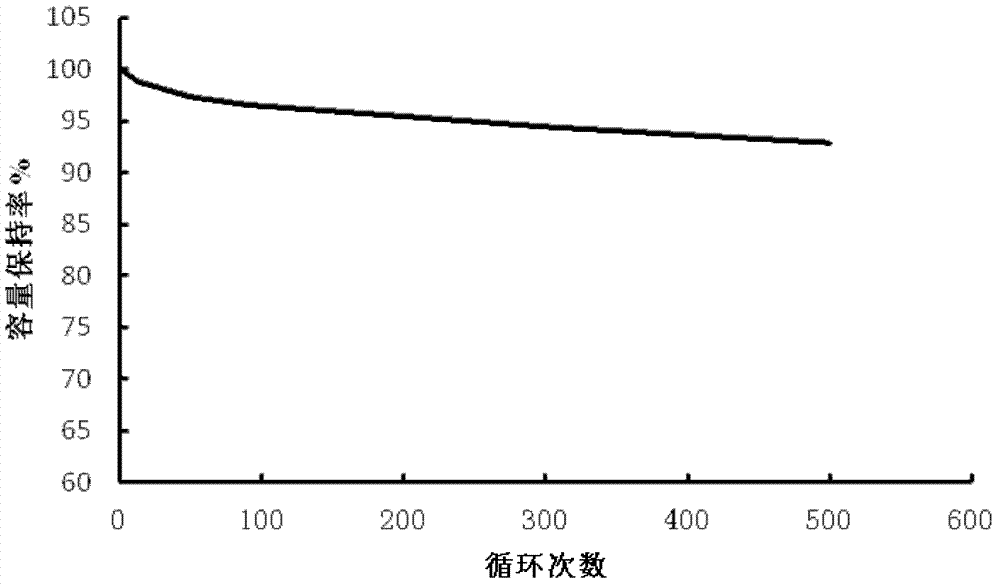
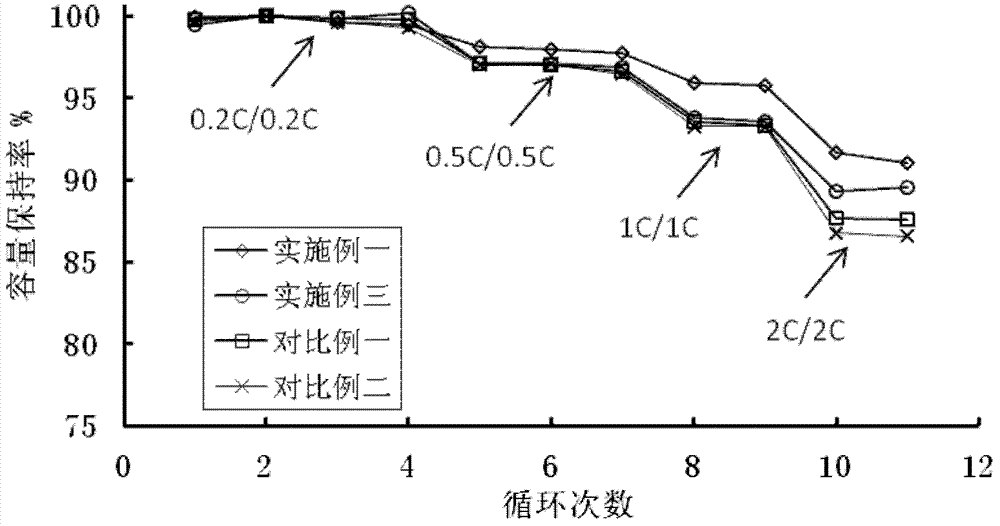
Preparation method of cathode material of LCO (lithium cobaltate)-based lithium ion battery
A technology for ion batteries and positive electrode materials, which is applied to battery electrodes, circuits, electrical components, etc., can solve the problems of battery safety hazards, fast charge and discharge rate of lithium cobalt oxide, etc., and achieve volumetric energy density, space utilization, and The effect of compaction density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] According to the ratio of lithium, cobalt, and titanium atoms being 1.05:1.00:0.01, cobalt carbonate, lithium carbonate, and titanium dioxide were ball milled and mixed for 5 hours; the mixed raw materials were placed in a roasting furnace, and roasted at 1000° C. for 12 hours in an air atmosphere; The calcined product is coarsely crushed, and then finely crushed using a jet mill. The average particle size D50 of the powder is 15 μm.
[0027] According to the ratio of lithium, cobalt, and titanium atoms being 1.01:1.00:0.01, cobalt carbonate, lithium carbonate, and titanium dioxide were ball milled and mixed for 5 hours; the mixed raw materials were placed in a roasting furnace, and roasted at 1000° C. in an air atmosphere for 12 hours; The calcined product is coarsely crushed, and then finely crushed using a jet mill. The average particle size D50 of the powder is 5 μm.
[0028]Weigh the powder with D50 of 15 μm and D50 of 5 μm according to the weight ratio of 1:1, add...
Embodiment 2
[0034] According to the ratio of lithium, cobalt and zirconium being 1.05:1.00:0.01, lithium carbonate, cobalt tetroxide and zirconium dioxide were ball milled and mixed for 5 hours; the mixed raw materials were placed in a roasting furnace, and roasted at 1000° C. in an air atmosphere for 12 hours; The calcined product is coarsely crushed, and then finely crushed using a jet mill. The average particle size D50 of the powder is 15 μm.
[0035] According to the ratio of lithium, cobalt and zirconium being 1.01:1.00:0.01, lithium carbonate, cobalt tetroxide and zirconium dioxide were ball milled and mixed for 5 hours; the mixed raw materials were placed in a roasting furnace, and roasted at 1000° C. in an air atmosphere for 12 hours; The calcined product is coarsely crushed, and then finely crushed using a jet mill. The average particle size D50 of the powder is 5 μm.
[0036] The powders with D50 of 15 μm and D50 of 5 μm are weighed according to the weight ratio of 1:1, and the...
Embodiment 3
[0039] Lithium carbonate, cobalt tetroxide and alumina were ball milled and mixed for 5 hours according to the ratio of lithium, cobalt and aluminum at a ratio of 1.05:1.00:0.01; the mixed raw materials were placed in a roasting furnace, and roasted at 1000° C. in an air atmosphere for 12 hours; The calcined product is coarsely crushed, and then finely crushed using a jet mill. The average particle size D50 of the powder is 15 μm.
[0040] Lithium carbonate, cobalt tetroxide and alumina are ball milled and mixed for 5 hours according to the ratio of lithium, cobalt and aluminum in a ratio of 1.01:1.00:0.01; the mixed raw materials are placed in a roasting furnace, and roasted at 1000° C. in an air atmosphere for 12 hours; The calcined product is coarsely crushed, and then finely crushed using a jet mill. The average particle size D50 of the powder is 5 μm.
[0041] The powders with D50 of 15 μm and D50 of 5 μm are weighed according to the weight ratio of 2:1, and then micron-s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Median particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com