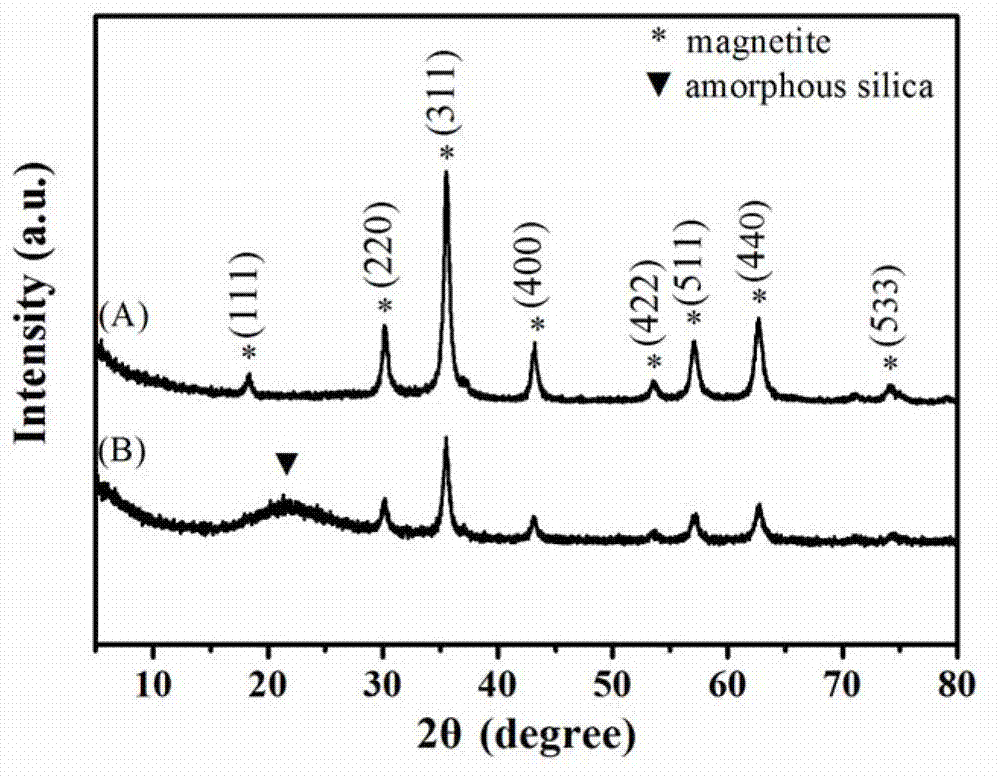
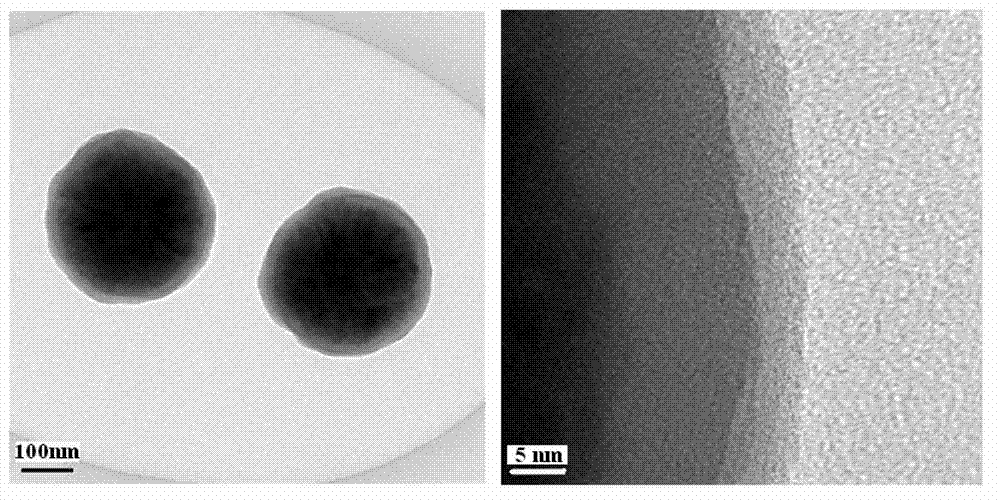
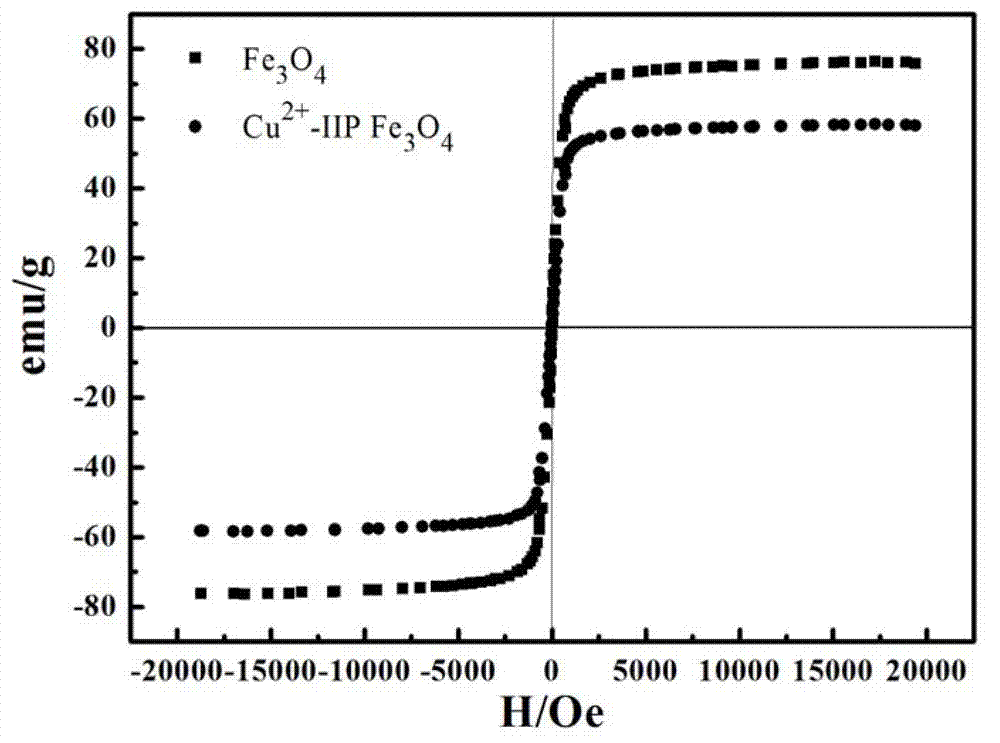
Preparation method of magnetic copper ion imprinting silica gel material
A technology of copper ions and magnetic nanoparticles, which is applied in the field of preparation of copper ion imprinted materials, can solve problems such as poor stability and small specific surface area, achieve good stability, increase specific surface area, and improve ion adsorption and exchange efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] First, 10g FeCl 3 ·6H 2 0 and 27g of sodium acetate were stirred and dissolved in 411g of ethylene glycol, and the resulting solution was placed in a hydrothermal kettle and reacted for 8h at 200°C; after the reaction, it was cooled to room temperature, and "magnetic separation collection, ethanol washing process" was repeated several times. Black magnetic Fe can be obtained after vacuum drying 3 o 4 Nanoparticles. Subsequently, weigh the 1g Fe prepared by the above steps 3 o 4 Nanoparticles were dispersed in a mixed solution containing 474g ethanol, 800g water, and 9g ammonia water, and 0.3g TEOS was added to the reaction system under stirring at room temperature, vigorously stirred for 6 hours, and then refluxed for 12 hours; Separation and collection, ethanol washing, and deionized water washing process" were repeated three times, and after vacuum drying, brown-black magnetic nanoparticles Fe with a silicon shell coating were obtained. 3 o 4 SiO 2 . Finally,...
Embodiment 2
[0026] First, 20g FeCl 3 ·6H 2 0 and 54g of sodium acetate were stirred and dissolved in 822g of ethylene glycol, and the resulting solution was placed in a hydrothermal kettle and reacted for 8h at 200°C; after the reaction was finished, it was cooled to room temperature, and "magnetic separation collection, ethanol washing process" was repeated several times. Black magnetic Fe can be obtained after vacuum drying 3 o 4 Nanoparticles. Subsequently, weigh 2 g Fe 3 o 4 Nanoparticles were dispersed in a mixed solution containing 948g ethanol, 1600g water and 18g ammonia water, and 0.6g TEOS was added to the reaction system under stirring at room temperature, vigorously stirred for 6 hours, and then refluxed for 6 hours; The collection, ethanol washing, and deionized water washing processes were repeated three times, and after vacuum drying, brown-black magnetic nanoparticles Fe with a silicon shell coating were obtained. 3 o 4 SiO 2 . Finally, put 1gFe in the three-neck ...
Embodiment 3
[0028] First, 5g FeCl 3 ·6H 2 O and 13.5g of sodium acetate were stirred and dissolved in 205.5g of ethylene glycol, and the resulting solution was placed in a hydrothermal kettle and reacted for 8h at 200°C; after the reaction, it was cooled to room temperature, and the "magnetic separation collection, ethanol washing process" was repeated several times. times, black magnetic Fe can be obtained after vacuum drying 3 o 4 Nanoparticles. Subsequently, weigh 0.5 g Fe 3 o 4 Nanoparticles were dispersed in a mixed solution containing 237g ethanol, 400g water and 4.5g ammonia water, and 0.15g TEOS was added into the reaction system under stirring at room temperature, vigorously stirred for 6h, and then refluxed for 12h; Separation and collection, washing with ethanol, and washing with deionized water were repeated three times, and after vacuum drying, brown-black magnetic nanoparticles Fe with a silicon shell coating were obtained. 3 o 4 SiO 2 . Finally, put 1gFe in the thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturated adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com