Preparation method for polystyrene/polyvinylidene fluoride cation exchange membrane
A polyvinylidene fluoride, cation exchange technology, applied in chemical instruments and methods, membrane technology, semi-permeable membrane separation, etc., can solve the problems of poor product stability, poor proton conductivity, high production cost, and achieve reduced membrane resistance, Good chemical stability and thermal stability, the effect of eliminating structural defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
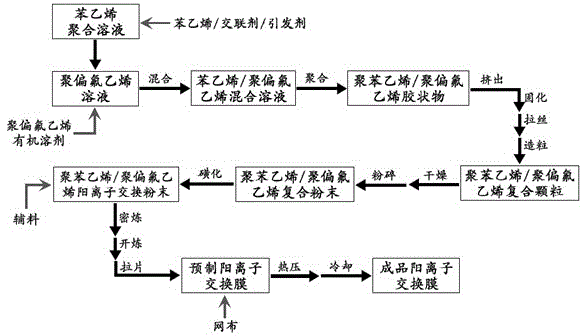
[0029]In a 500ml three-neck flask, add 50g of polyvinylidene fluoride powder (brand DF-1), add 180ml of dimethylacetamide, heat to 85°C, and stir for 90 minutes to form a transparent polyvinylidene fluoride solution. Take a 250 ml Erlenmeyer flask, add 36.5 g of styrene, 3.5 g of divinylbenzene (80.5% content) and 1.8 g of benzoyl peroxide, and stir magnetically at room temperature until the solid is completely dissolved to obtain a styrene polymerization solution. Slowly add the styrene polymerization solution dropwise into the high-speed stirred polyvinylidene fluoride solution, control the addition within 20 minutes, and then continue stirring for 30 minutes to obtain a homogeneous mixed solution.
[0030] Subsequently, the above mixed solution was poured into clean watch glasses in batches, wrapped and sealed with plastic wrap, placed in an oven, pre-polymerized at 75°C for 2 hours, and polymerized at 85°C for 12 hours. Take out the watch glass, take out the polymerized po...
Embodiment 2
[0035] 45.5 grams of styrene, 4.5 grams of divinylbenzene (80.5%) and 2.0 grams of benzoyl peroxide were used to prepare a styrene polymerization solution to replace the styrene polymerization solution in Example 1, and the remaining proportions remained unchanged. According to the same method as in Example 1, a finished polystyrene / polyvinylidene fluoride cation exchange membrane was prepared.
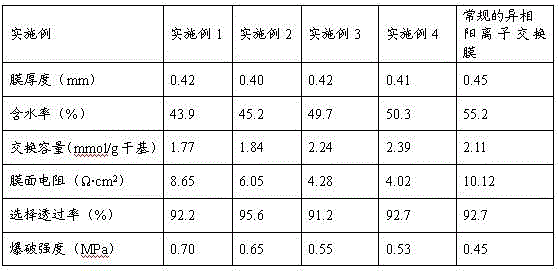
[0036] According to the determination method described in the national standard (HY / T 034.2-1994), the performance of the prepared cation exchange alloy membrane was measured, and the results are shown in Table 1. Membrane resistance is 40% lower than conventional polystyrene-based cation exchange heterogeneous membranes, and membrane strength is increased by 44%.
Embodiment 3
[0038] 55.2 grams of styrene, 4.8 grams of divinylbenzene (80.5%) and 2.0 grams of benzoyl peroxide were used to prepare a styrene polymerization solution to replace the styrene polymerization solution in Example 1, and the remaining proportions remained unchanged. According to the same method as in Example 1, a finished polystyrene / polyvinylidene fluoride cation exchange membrane was prepared.
[0039] According to the determination method described in the national standard (HY / T 034.2-1994), the performance of the prepared cation exchange alloy membrane was measured, and the results are shown in Table 1. The membrane resistance is 58% lower than that of conventional polystyrene-based cation exchange heterogeneous membranes, which is very close to that of cation exchange homogeneous membranes (generally, the membrane resistance of cation exchange homogeneous membranes is 2~4 Ω·cm 2 ); the membrane strength is 22% higher than that of conventional heterogeneous membranes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com