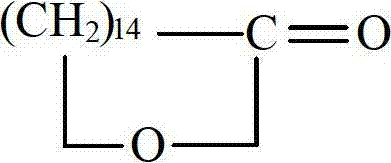
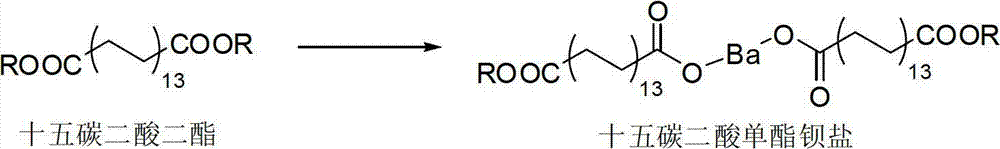Synthesis method for pentadecanoicacid
A cyclopentadecanolactone and synthesis method technology, applied in the field of compound synthesis, can solve problems such as immature process conditions, harsh production conditions, and long process flow, and achieve strong industrial operability, low environmental pollution, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The synthetic method of cyclopentadecanolide comprises the following steps:
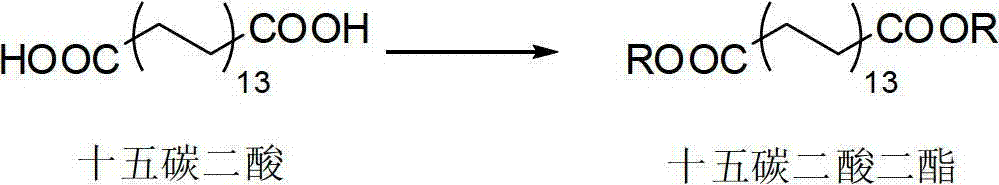
[0045] (1) Forming pentadecanedioic acid diester from the reaction of pentadecanedioic acid;
[0046]
[0047] (2) Synthesize pentadecanedioic acid monoester salt from the pentadecanedioic acid diester obtained in step (1);
[0048]
[0049] (3) The pentadecanedioic acid monoester salt obtained in step (2) is acidified and treated to obtain pentadecanedioic acid monoester;
[0050]
[0051] (4) generating 15-hydroxypentadecanoic acid by reducing the ester group of the pentadecanedioic acid monoester obtained in step (3);
[0052]
[0053] (5) The 15-hydroxypentadecanoic acid solid obtained in step (4) is subjected to a bromination reaction to obtain 15-bromopentadecanoic acid;
[0054]
[0055] (6) Dissolve quantitative 15-bromopentadecanoic acid in an organic solvent, add a small amount of iodine, and perform a ring closure reaction to obtain crude pentadecalactone;
[0056]...
Embodiment 1
[0060] Mix 27.2 g of pentadecanedioic acid with 64 ml of n-butanol, add 4 g of sodium bisulfate and 10 ml of cyclohexane under stirring at 115° C., and the reaction time is 4 hours. After filtering and washing with water until neutral, the organic solvent was distilled off to obtain 34.7 g of dibutyl pentadecanoic acid. Dissolve 25g of barium hydroxide in methanol, slowly add it dropwise to 30g of dibutyl pentadecanedioate at room temperature, react for 14 hours, filter, wash with water, and generate pentadecanedioic acid monoester barium salt. After diluting with a small amount of water, the solution was adjusted to a pH of 2 with dilute hydrochloric acid, extracted with ethyl acetate, washed with water until neutral, and evaporated to remove the organic solvent to obtain 21.2 g of monobutyl pentadecanedioic acid as a solid.
[0061] At 0°C, weigh 10 g of monobutyl pentadecanedioic acid and dissolve it in 200 ml of methanol solution, add 3.3 g of sodium borohydride in batches...
Embodiment 2
[0065] Mix 27.2 g of pentadecanedioic acid with 50 ml of n-butanol, add 4 g of sodium bisulfate and 10 ml of cyclohexane with stirring at 110° C., and the reaction time is 6 hours. After filtering and washing with water until neutral, the organic solvent was distilled off to obtain 34.7 g of dibutyl pentadecanoic acid. Dissolve 18g of barium hydroxide in a mixed solution of methanol and water, slowly add it dropwise to 30g of dibutyl pentadecanedioate at room temperature, react for 24 hours, filter, wash with water, and generate pentadecanedioic acid mono ester barium salt. After diluting with a small amount of water, the solution was adjusted to a pH of 2 with dilute hydrochloric acid, extracted with toluene, washed with water until neutral, and evaporated to remove the organic solvent. 20.8 g of white pentadecanedioic acid monobutyl was obtained.
[0066] At 0°C, 10 g of monobutyl pentadecanedioic acid was weighed and dissolved in 200 ml of ethanol solution, and 3.3 g of so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com