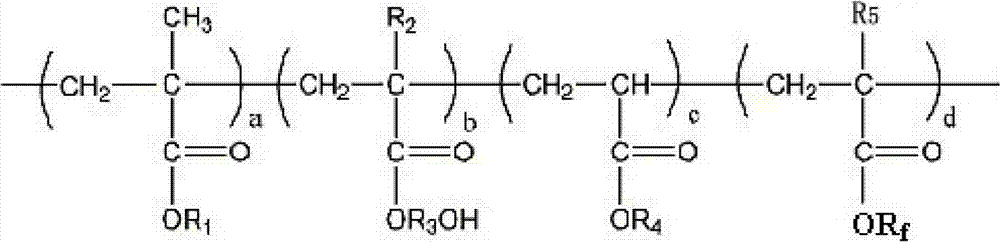
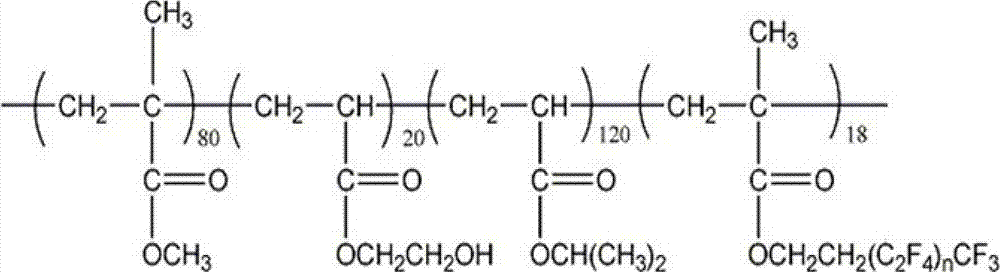
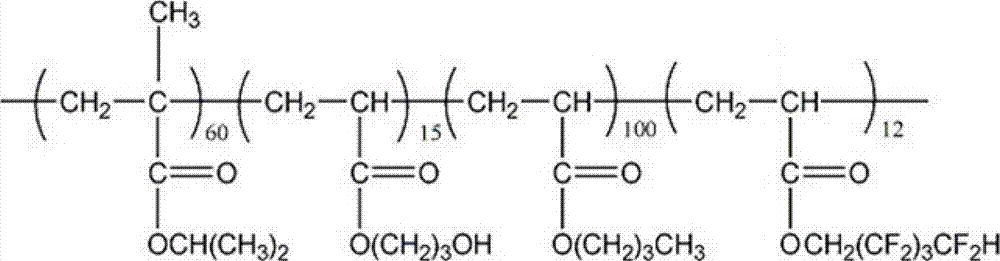
Cross-linked fluorine (methyl)-containing acrylic segmented copolymer and its preparation method and use
A block copolymer, cross-linking technology, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problem that the film performance and hydrophobicity cannot be well balanced at the same time, so as to improve the poor performance and improve the coating performance. Membrane performance, the effect of expanding the range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of macromolecular initiator: the methyl methacrylate of 3g, the isopropyl acrylate of 6g, the hydroxyethyl acrylate of 1.5g and 6.5g dimethylbenzene join together in the reaction eggplant bottle and mix and dissolve, then seal the reaction bottle, Freeze the system with liquid nitrogen-vacuumize-thaw three times; under the protection of argon, add 0.048g ruthenium dichloride, 0.02g copper powder and 0.12g tris(2-pyridylmethyl)amine to seal the reaction bottle, and then freeze the system with liquid nitrogen-vacuumize-thaw-argon, repeat three times; finally put the reaction eggplant bottle in a 30°C oil bath, add 0.06g of carbon tetrachloride, and electromagnetically stir for 6 hours. Then add tetrahydrofuran to dilute, and then go through neutral alumina column chromatography. After most of the solvent evaporates, the prepared polymer is precipitated with petroleum ether and vacuum-dried to obtain a macromolecular initiator.
[0046] Preparation of cross...
Embodiment 2
[0050] Preparation of macroinitiator: 3.5g of isopropyl methacrylate, 6g of butyl acrylate, 1.5g of hydroxypropyl acrylate and 6g of dimethylformamide were added to the reaction eggplant bottle for mixing and dissolving, then sealed Reaction bottle, freeze the system with liquid nitrogen-vacuumize-thaw, repeat three times; under the protection of argon, then add 0.12g nickelous chloride, 0.01g nickel powder and 0.25g 3,5 Picolidinedicarboxylic acid, seal the reaction bottle, and then freeze the system with liquid nitrogen-vacuumize-thaw, repeat three times; finally put the reaction eggplant bottle in a 50°C oil bath, add 0.15g α-bromopropionitrile, electromagnetically stir for 10 hours, then add tetrahydrofuran to dilute, and then go through neutral alumina column chromatography. After most of the solvent volatilizes, the prepared polymer is precipitated with petroleum ether and vacuum-dried to obtain a macromolecular initiator.
[0051] Preparation of cross-linked fluorine-c...
Embodiment 3
[0055] Preparation of macromolecular initiator: 4g of tert-butyl methacrylate, 6.5g of isoamyl acrylate, 2g of hydroxybutyl acrylate and 7.25g of anisole were added to the reaction eggplant bottle for mixing and dissolving, then sealed for reaction bottle, freeze the system with liquid nitrogen-vacuumize-thaw, repeat three times; under the protection of argon, then add 0.26g cuprous chloride, 0.05g copper powder and 0.5g 2,5-thiophenedicarboxylic acid, seal the reaction bottle , and then freeze the system with liquid nitrogen-vacuumize-thaw-argon, repeat three times; finally put the reaction eggplant bottle into a 70°C oil bath, add 0.3g of initiator α-iodoisobutyronitrile, electromagnetic Stir for 15 hours, then add tetrahydrofuran to dilute, and then go through neutral alumina column chromatography. After most of the solvent volatilizes, the prepared polymer is precipitated with petroleum ether and vacuum-dried to obtain a macromolecular initiator.
[0056] Preparation of cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com