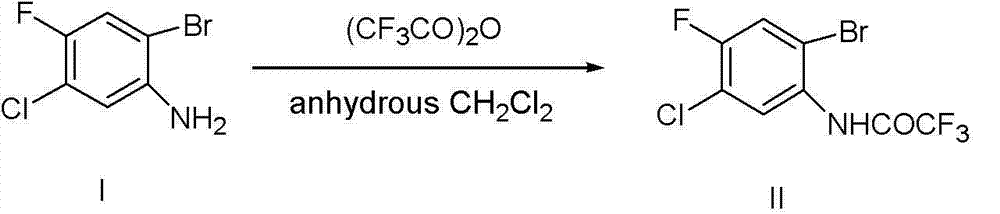Synthetic method of oxidation indoles compound
A technology for the synthesis of oxindole and its synthesis method, which is applied in the field of synthesis of oxindole compounds, can solve the problems of unsatisfactory chemical yield and complex operation, and achieve good application prospects, simple treatment process, and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
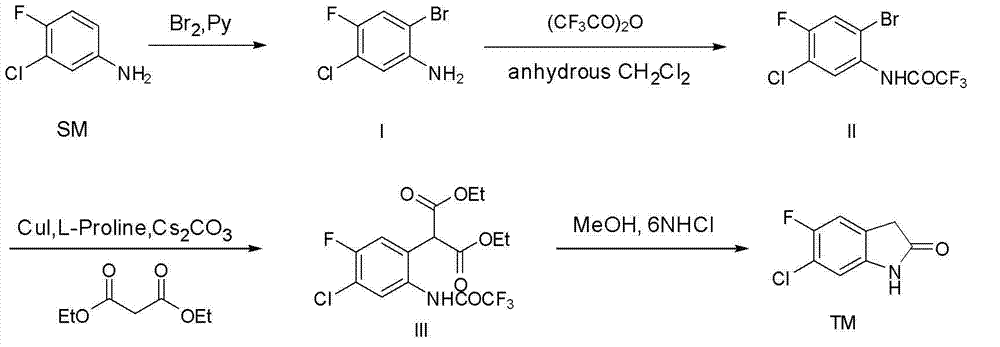
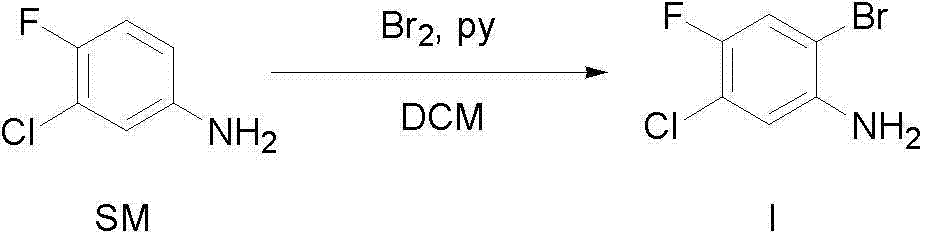
[0020]
[0021] Under stirring at 0-5°C, a solution of 109.8 g of liquid bromine (MW=159.81,687 mmol, 1 eq.) in dichloromethane (200 mL) was added dropwise to a solution containing 3-chloro-4-fluoro 100 g of aniline (MW=145.56, 687 mmol, 1eq.), and 80.4 g of pyridine (MW=79.10, 1.02 mol, 1.5eq. ) in dichloromethane (600 mL) were placed in a 3L three-neck round bottom flask. After the dropwise addition, keep the temperature at 0-5°C and continue to stir for one hour, and follow the TLC to complete the reaction. The reaction solution was washed with water (3×200 mL) and saturated brine (1×200 mL), dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a crude oily product. Recrystallized from n-heptane to obtain 60.38 g of white crystals with a yield of 39%. HPLC purity: 99.65%; 1 H NMR (400 MHz CDCl 3 ): δ 3.91(2H, br. s), 6.71(1H,d), 7.16(1H,d).
Embodiment 2
[0023]
[0024] In a 1L three-neck round bottom flask, add 2-bromo-5-chloro-4-fluoroaniline (I) 40 g (MW=224.46, 178.2 mmol, 1 eq.) and anhydrous reagent dichloromethane 300 mL. At 0°C, 44.9 g of trifluoroacetic anhydride (MW=210.03, 213.8 mmol, 1.2 eq.) was added dropwise, and the stirring was continued for 1 hour. TLC detected that the reaction was complete. Neutralize with saturated sodium bicarbonate solution, separate the organic phase, extract the aqueous phase with dichloromethane (3×100 mL), combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate to dryness to give a white solid 55.96 g, yield 98%. HPLC purity: 97.90%; 1 H NMR (400 MHz CDCl 3 ): δ 7.44(1H, d), 8.305(1H,br. s), 8.44(1H,d).
Embodiment 3
[0026]
[0027] In a 500 mL three-neck round bottom flask, N 2 Under protection, 20.0 g (MW=320.47, 62.4 mmol, 1 eq.) of 2-bromo-5-chloro-4-fluorotrifluoroacetanilide (II) was dissolved in 250 mL of anhydrous DMSO, and malonic acid was added at room temperature Diethyl ester 12.0 g (MW=160.17, 74.88mmol, 1.2eq.), cuprous iodide 2.4 g (MW=190.45, 12.4mmol, 0.2eq.), L-proline 2.88 g (MW=115.13, 24.96 mmol, 0.4eq.), 81.4 g cesium carbonate (MW=325.82, 249.6 mmol, 4eq.). Stir at 50°C for 4 hours, and follow the completion of the reaction by TLC. Saturated ammonium chloride solution was added, and the blue precipitate disappeared. Extracted with ethyl acetate (3×100 mL), combined the organic layers, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated. The obtained pale yellow oily crude product was dissolved in 200 mL of methanol and 200 mL of 6N HCl aqueous solution, and reacted at 80° C. for 2 to 3 hours, and the reaction was compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com