Binary inactivated vaccine against Japanese encephalitis virus and porcine parvovirus and preparation method thereof
A dual inactivated vaccine, swine Japanese encephalitis technology, applied in antiviral agents, viral antigen components, pharmaceutical formulations, etc., can solve the problems of cumbersome operating procedures, affecting the quality of vaccines, and high cell loss rate, reducing clinical Effects of stress response, overcoming high rate of cell attrition, simplifying immunization program
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
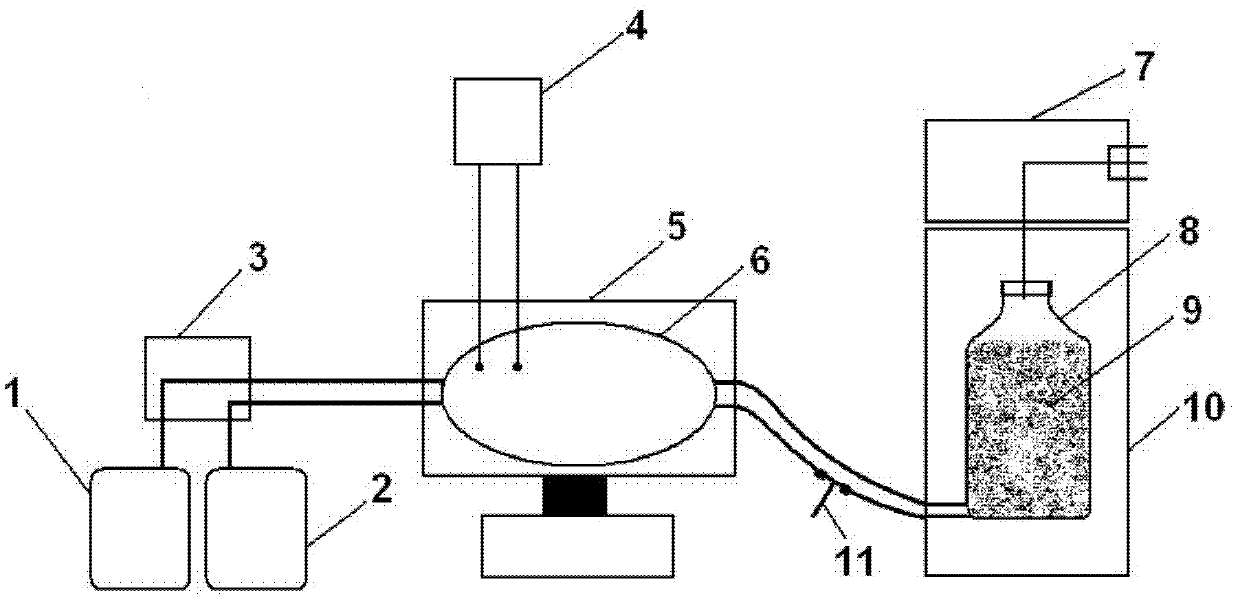
Image
Examples
preparation example Construction
[0073] (1) Cell seeding, adsorption and culture
[0074] Dilute the animal cell suspension at 0.5×10 6 ~5×10 6 The final density of cells / g carrier is inoculated in the carrier tank, and the parameters of the bioreactor are set to carry out the cell adsorption and culture process. Among them, animal cells for cultivating porcine Japanese encephalitis virus include BHK-21, IBRS-2, ST, Vero, C6 / 36 and chicken embryo fibroblasts, preferably BHK-21 cells. The cultured cells of porcine parvovirus include ST, PK-15, IBRS-2, CPK and MVPK cells, preferably ST cells.
[0075] (2) Virus inoculation, adsorption and proliferation
[0076] After the cells grow to 0.5×10 7 ~1.0×10 8 When the density of cells / g carrier is reached, replace the cell culture medium, then inoculate the porcine Japanese encephalitis virus and porcine parvovirus liquid into the carrier tank respectively, and set the parameters of the bioreactor to carry out the process of virus adsorption and multiplication. ...
Embodiment 1
[0097] The microcarrier used in this embodiment is polyester fiber, and the cell lines used for making seedlings are BHK-21 cells and ST cells, wherein, porcine Japanese encephalitis virus uses BHK-21 cell proliferation, and porcine parvovirus uses ST cell proliferation .
[0098] The bioreactor is a 10L carrier bottle Tidecell tidal bioreactor.
[0099] The formula of cell growth liquid: the growth liquid of BHK-21 cell is the DMEM (Invitrogen company) containing 5% (V / V) fetal bovine serum; The growth liquid of ST cell is the DMEM containing 5% (V / V) fetal bovine serum α-MEM (Invitrogen Corporation).
[0100] The formula of cell maintenance solution: the growth solution of BHK-21 cells is DMEM (Invitrogen Company) containing 1% (V / V) fetal bovine serum; the growth solution of ST cells is DMEM containing 1% (V / V) fetal bovine serum α-MEM (Invitrogen Corporation).
[0101] (1) Cell seeding, adsorption and culture
[0102] In a 10L bioreactor, add 650g of sterile microcarri...
Embodiment 2
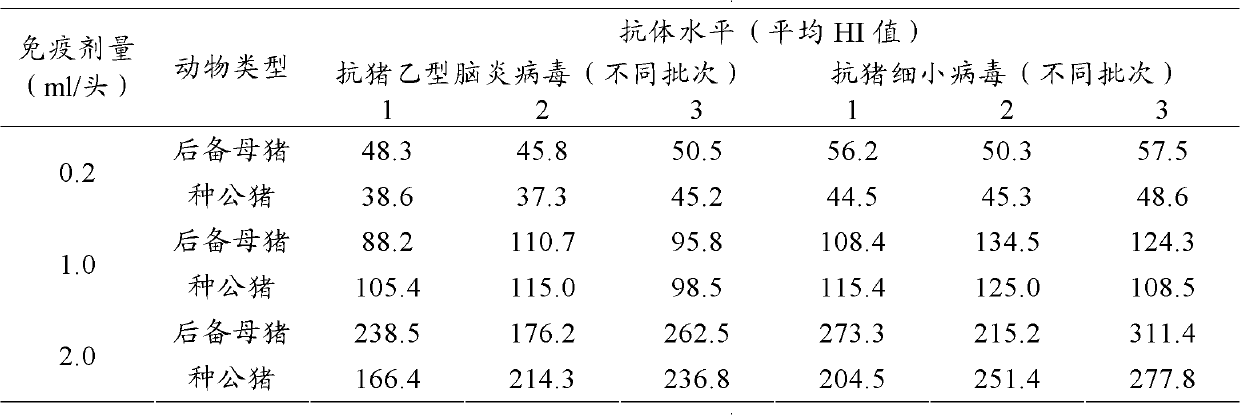
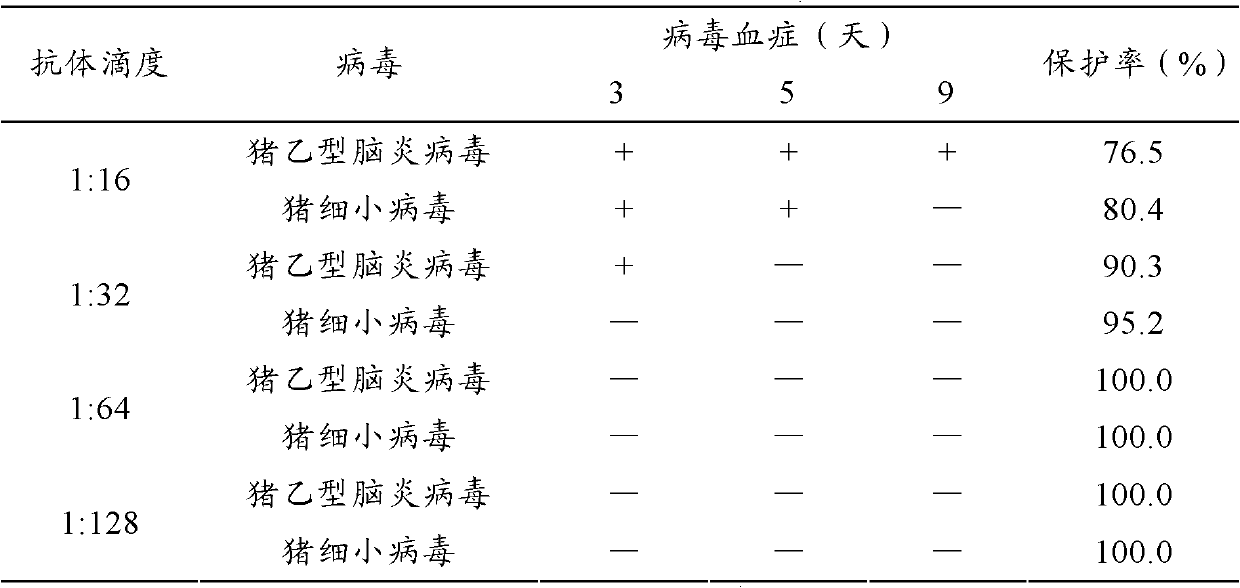
[0118] 1. Finished product inspection of dual inactivated vaccine:
[0119] Physical property test:
[0120] a) Appearance: milky white or light red homogeneous emulsion.
[0121] b) Dosage form: bi-directional, that is, water-in-oil-in-water (W / O / W). Take a clean straw, suck a small amount of vaccine and drop it in clean cold water, except for the first drop, it is in the shape of oil droplets and does not spread.
[0122] c) Stability: Take 10ml of the vaccine and put it into a centrifuge tube, centrifuge at 3,000rmp / min for 15min, no stratification occurs, and the water phase ≤0.5ml precipitates at the bottom of the tube; take the vaccine and place it at about 37°C for 21 days, the result shows no stratification, no Demulsification phenomenon.
[0123] d) Viscosity: use a 1ml clean straw (1.2mm lower diameter, 2.7mm upper diameter) to draw 1ml of vaccine at about 25°C to let it flow out vertically and naturally, and record the time required to flow out 0.4ml. The results...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Mean titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com