Battery
A battery and electrolyte technology, applied in the field of electrochemical energy storage, can solve the problems of restricting the development of batteries, not being able to meet them at the same time, and high technical thresholds, and achieve the effects of shortening the migration distance, solving the problem of diffusion resistance, and operating safely
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 2 approach
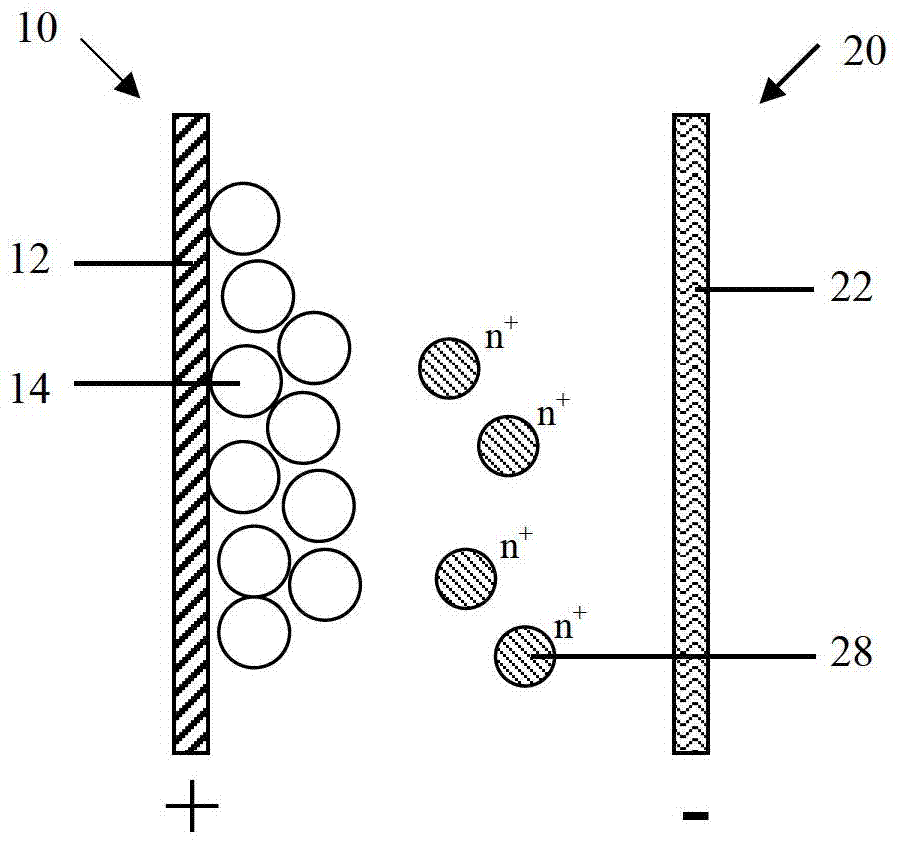
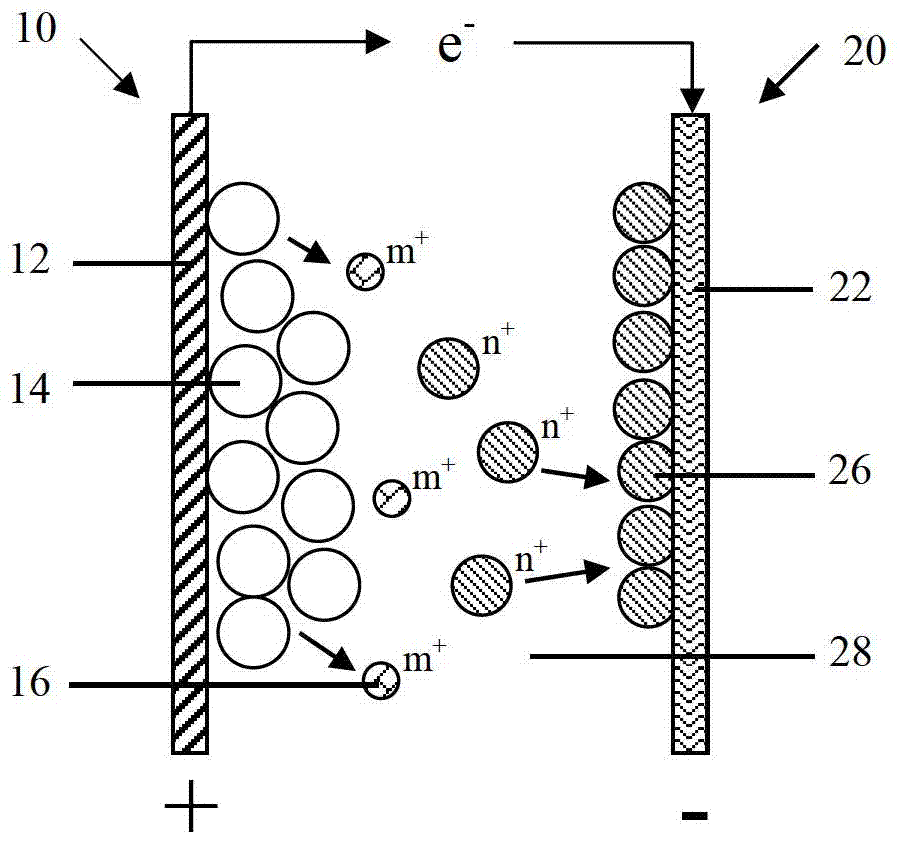
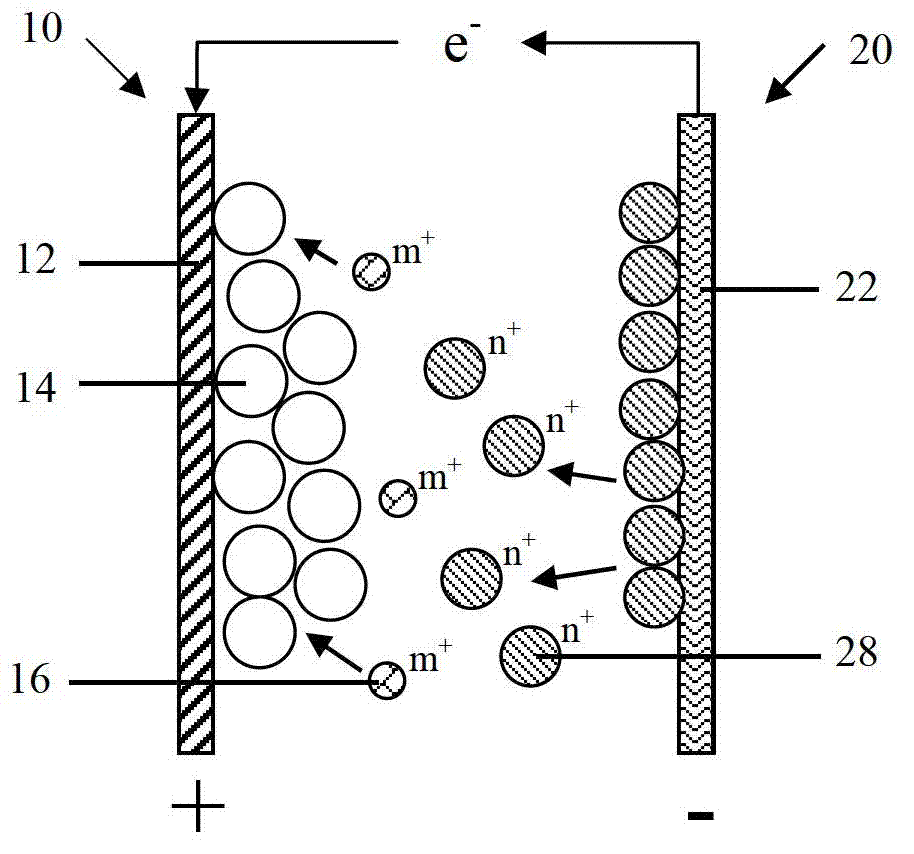
[0112] Please refer to Figure 4 As shown, the second embodiment of the present invention provides a battery, and the difference from the battery disclosed in the first embodiment is that in the second embodiment, the negative electrode 20 also includes a negative electrode active material 24 formed on the surface of the negative electrode current collector 22, The negative electrode active material 24 can be oxidized-dissolved into active ions 28 during the discharge process.
[0113] The negative electrode collector 22 is only used as a carrier for electron conduction and collection, and does not participate in the reaction of the negative electrode 20. The negative electrode active material 24 is formed on the negative electrode collector 22 by coating, electroplating or sputtering. The sputtering method includes but is not limited to magnetron sputtering. Specifically, the negative electrode current collector 22 is copper foil, the negative electrode active material 24 is...
no. 3 approach
[0144] The third embodiment of the present invention also discloses a battery. The difference from the battery disclosed in the second embodiment is that in the third embodiment, the negative electrode 20 only includes the negative electrode current collector 22, but the negative electrode current collector 22 not only conducts and collects electrons At the same time, it is also equivalent to that the negative active material can participate in the reaction of the negative electrode 20, and can be oxidized and dissolved into the active ion 28 during the battery discharge process, that is, the material of the negative electrode current collector 22 is the same as the elemental material of the active ion 28, for example: active ion 28 is zinc ion, and the corresponding negative electrode current collector 22 is metallic zinc.
[0145] In the third embodiment, the negative electrode 20 includes the negative electrode current collector 22 participating in the electrochemical reacti...
Embodiment 1-1
[0152] Use stainless steel as the working electrode, the stainless steel type is 304, the zinc electrode is the counter electrode and the reference electrode, in the sulfate electrolyte 2mol / L ZnSO 4 and 2mol / L Li 2 SO 4 The electrochemical behavior of stainless steel was studied by cyclic voltammetry in the voltage range of 1.0-2.4V. Stainless steel is not passivated.
[0153] Figure 5 It is the cyclic voltammetry curve of the stainless steel 304 without passivation treatment in embodiment 1-1. It can be seen from the figure that a broad oxidation peak appeared at 1.9V (Vs.Zn) during the first anodic scan of stainless steel, followed by an obvious O 2 The precipitation peak is accompanied by the increase of current. In the subsequent cathodic scan, a relatively small reduction peak appeared at 1.4 V. The oxidation peak at 1.9V after 1 cycle was hindered, which means that an oxide layer was formed on the stainless steel surface in the first cycle, and the oxide layer in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com