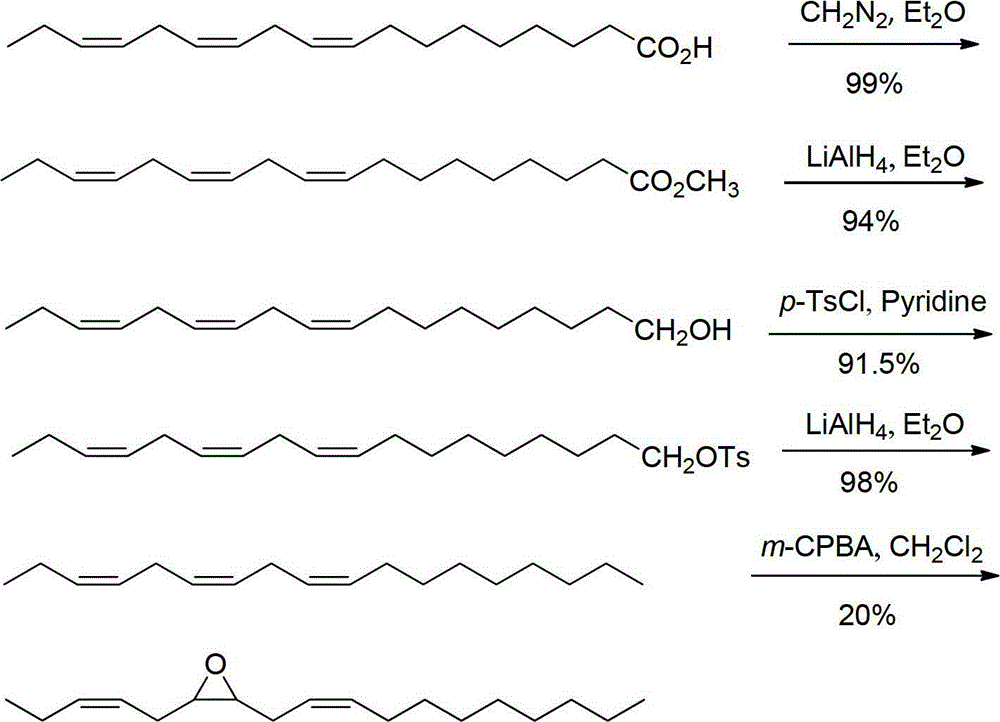
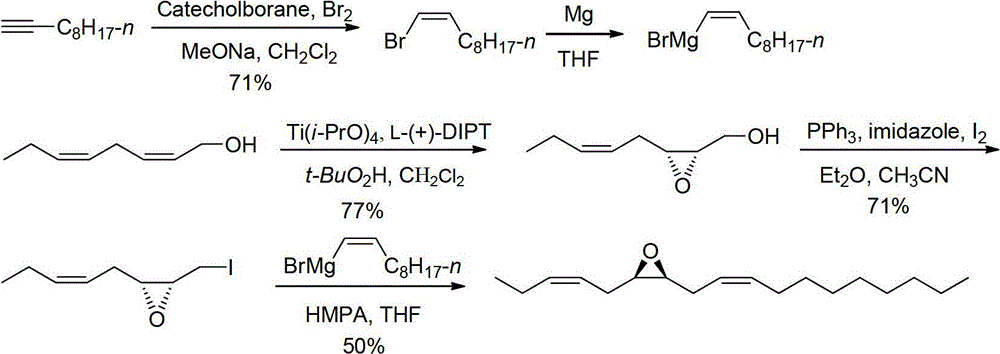
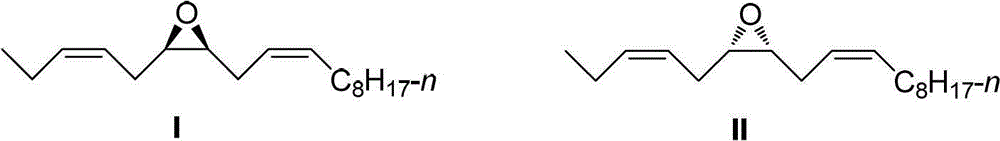Stereoselective synthetic method for tea geometrid sex pheromone
A technology of stereoselectivity and synthesis method, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high price, difficult purification, poor selectivity, etc., and achieve high enantioselectivity, simple separation and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Step 1 Synthesis of (Z)-4-chloro-2-buten-1-ol (2)
[0042] Dissolve cis-2-butene-1,4-diol (1) (22g, 250mmol) in tetrahydrofuran (120mL) solvent, add pyridine (22mL, 275mmol) at 0°C, drop through constant pressure dropping funnel Thionyl chloride (20 mL, 275 mmol) was added, raised to room temperature, and stirred for 1 day. After the reaction was quenched by adding 50 mL of ice water dropwise, the liquid was separated and the aqueous phase was extracted with diethyl ether (3×30 mL). The organic phases were combined, washed with 10% sodium hydroxide solution (30mL), saturated sodium bicarbonate solution (30mL) and saturated brine (20mL), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and purified to obtain compound 2 (18.5g, 70%). Colorless liquid, b.p.78-82°C (13mmHg). 1 H NMR (400MHz, CDCl 3 )δ2.58(s,1H),4.10(d,J=7.0Hz,2H),4.24(d,J=5.6Hz,2H),5.70-5.80(m,2H)ppm; 13 C NMR (100MHz, CDCl 3 ) δ39.0, 57.9, 127.3, 133.1ppm; MS (ESI) ...
Embodiment 2
[0062] Step 1 Synthesis of (Z)-4-chloro-2-buten-1-ol (2)
[0063] Dissolve cis-2-butene-1,4-diol (1) in ether solvent, add pyridine at 0°C, add thionyl chloride dropwise through a constant pressure dropping funnel, rise to room temperature, and stir for 1 day . After the reaction was quenched by adding ice water dropwise, the mixture was allowed to stand for liquid separation, and the aqueous phase was extracted with diethyl ether. The combined organic phases were dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and purified to obtain a colorless liquid 2 (68%).
[0064] Step 2 Synthesis of (Z)-2-en-5-yne tetradec-1-alcohol (3)
[0065] Anhydrous potassium carbonate, cuprous iodide, and sodium iodide were added to N,N-dimethylacetamide solvent, 1-decyne was added at room temperature, stirred for 1 h, compound 2 was added dropwise, and stirred at room temperature for 6 h. After stopping the reaction, filter, concentrate under reduced pressu...
Embodiment 3
[0083] Step 1 Synthesis of (Z)-4-chloro-2-buten-1-ol (2)
[0084] Dissolve cis-2-butene-1,4-diol (1) in tetrahydrofuran solvent, add pyridine at room temperature, add thionyl chloride dropwise through a constant pressure dropping funnel, raise the temperature to 65°C, and stir for 10 hours. After adding ice water to quench the reaction, let stand to separate the layers, and extract the aqueous phase with diethyl ether. The combined organic phases were dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and purified to obtain compound 2 (66%).
[0085] Step 2 Synthesis of (Z)-2-en-5-yne tetradec-1-alcohol (3)
[0086] Anhydrous cesium carbonate, cuprous iodide, and sodium iodide were added to N,N-dimethylformamide solvent, 1-decyne was added at room temperature, stirred for 1 h, compound 2 was added dropwise, and stirred at room temperature for 8 h. After stopping the reaction, filter, concentrate under reduced pressure, dilute with water, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com