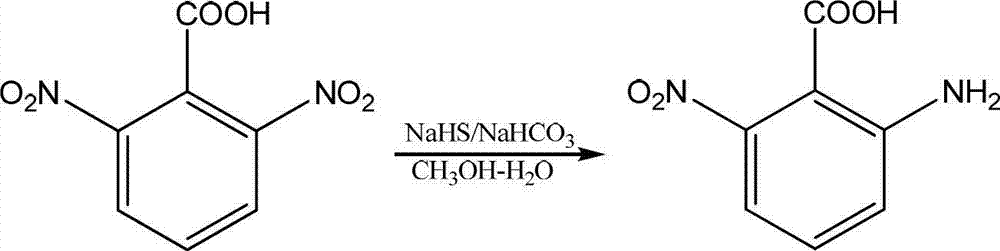
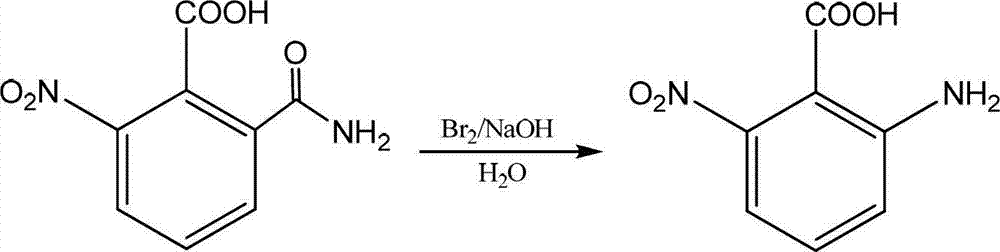
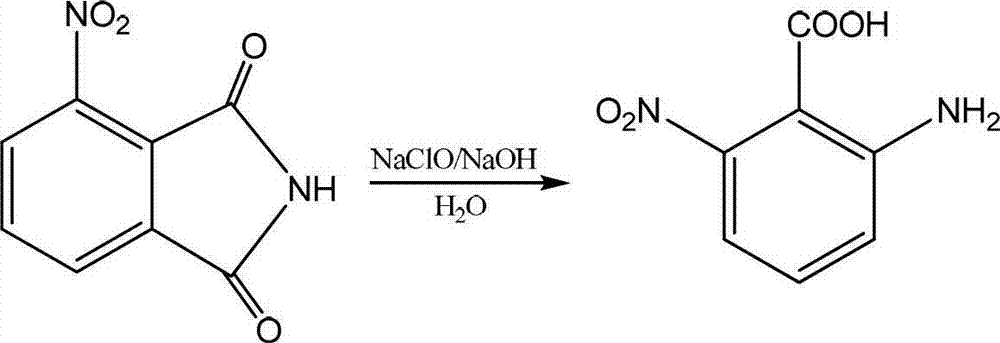Preparation method of 2-amino-6-nitrobenzoic acid
A technology of nitrobenzoic acid and amino, which is applied in the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc. It can solve the problems of difficult synthesis of ammonolysis process, difficult preparation of raw materials, difficult acquisition, etc., and achieve product purity Good, simple post-processing operation, and reduced side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]Put 20.3g of 2-chloro-6-nitrobenzoic acid, 1.2g of cuprous oxide, 40g of cesium carbonate, 50g of DMF and 85g of 30% self-mixed ammonia into a 500mL autoclave. Raise the temperature to 90°C, pass ammonia gas during the reaction to maintain the pressure in the kettle at 0.7-0.8 MPa, and react for 12 hours. Sampling control. The phases were separated and the upper layer was collected. Add hydrochloric acid to acidify to pH 1-2. Filter and collect the filtrate. Add diethyl ether to extract three times, and collect the upper diethyl ether phase. Distillation and desolvation gave 18.7 g of A-NBA crude product (HPLC content of A-NBA: 88%), converted yield: 90%. Recrystallization was carried out with ethyl acetate to obtain 15.5 g of A-NBA product (HPLC purity>97%), the total yield: 85%. 1 HNMR (DMSO-d 6 ,400MHz): δ6.9(1H,d,J=8Hz), δ7.0(1H,d,J=8Hz), δ7.3(1H,t,J=8Hz), δ8.7(2H,s ). ESI-MS m / z: 181.0221[M-H] - .
Embodiment 2
[0045] Put 29.6g of 2-iodo-6-nitrobenzoic acid, 1.9g of cuprous iodide, 100g of ethanol and 68g of 30% self-made ammonia into a 500mL autoclave. The temperature was raised to 100°C, and the reaction was carried out for 15 hours. During the reaction, the system pressure dropped from 1.1 MPa to 0.8 MPa. Sampling control. Add hydrochloric acid to acidify to pH 2-3. Add ethyl acetate to extract three times, and collect the ethyl acetate phase. Under reduced pressure, 18.8 g of crude A-NBA was obtained (HPLC content of A-NBA: 89%), and the converted yield was 92%. Recrystallization was carried out with ethyl acetate / petroleum ether mixed solvent to obtain 15.8 g of A-NBA product (HPLC purity > 97%), and the total yield: 87%. 1 HNMR (DMSO-d 6 ,400MHz): δ6.9(1H,d,J=8Hz), δ7.0(1H,d,J=8Hz), δ7.3(1H,t,J=8Hz), δ8.7(2H,s ). ESI-MS m / z: 181.0221[M-H] - .
Embodiment 3
[0047] Put 24.8g of 2-bromo-6-nitrobenzoic acid, 1.2g of cuprous bromide, 100g of isopropanol and 68g of 30% self-made ammonia into a 500mL autoclave. The temperature was raised to 105°C, and the reaction was carried out for 15 hours. During the reaction, the system pressure dropped from 1.2MPa to 0.9MPa. Sampling control. Add sulfuric acid to acidify to pH 1-2. Add ethyl acetate to extract three times, and collect the ethyl acetate phase. Under reduced pressure, 18.4 g of crude A-NBA was obtained (HPLC content of A-NBA: 90%), and the converted yield was 91%. Recrystallization was carried out with ethyl acetate / hexane mixed solvent to obtain 15.7 g of A-NBA product (HPLC purity > 97%), and the total yield: 86%. 1 HNMR (DMSO-d 6 ,400MHz): δ6.9(1H,d,J=8Hz), δ7.0(1H,d,J=8Hz), δ7.3(1H,t,J=8Hz), δ8.7(2H,s ). ESI-MS m / z: 181.0221[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com